
Primary adrenal tuberculosis infection in patients with Behcet’s disease presenting as isolated adrenal metastasis by 18F-FDG PET/CT: a rare case report and literature review
Introduction
Adrenal TB, the fifth most common cause of EPTB after tuberculosis of the liver, spleen, kidney and bone (1), is rare and represents between 1–2% of the etiologies of adrenal masses (2), and it can be isolated or coexist with pulmonary tuberculosis (PTB). Adrenal involvement was discovered in 6% of the autopsy series of patients with active tuberculosis (1). Adrenal TB is an essential etiology of Addison’s disease, especially in developing countries (3). However, Addison’s disease does not appear until more than 90% of the gland is destroyed (4). Furthermore, adrenal TB patients generally have a history of PTB, but nearly 12% of adrenal TB patients may have no extra-adrenal TB (5).
In recent years, advances in the diagnosis of tuberculosis have been seen with the development of bacterial product detection and imaging evidence. The main diagnostic tests for tuberculosis are sputum smear microscopy, rapid molecular tests, and culture-dependent methods. The molecular testing of the Xpert MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) recommended by WHO can simultaneously detect tuberculosis (better accuracy than smear microscopy) and drug resistance (rifampicin). Culture-based methods are the accepted reference standard, but these take much more intricate lab capacity and require up to 3 months to obtain the results. In addition, the urinary lipoarabinomannan (LAM) test is presently recommended for seriously ill and hospitalized patients with human immunodeficiency virus (HIV) and CD4 ≤100 cells per µL. Chest X-ray is now endorsed by WHO for screening and diagnosing tuberculosis in some populations (6).
Adrenal TB is characterized by a distinctive clinical and radiological pattern, and it is challenging to obtain an accurate diagnosis even after biopsy. The clinical symptoms of adrenal TB are atypical and include adrenal incidentaloma and adrenal insufficiency with a unilateral or bilateral mass-like enlargement of adrenal glands (7). The imaging examinations usually lack specificity. The CT scans reveal enlarged adrenal glands when Mycobacterium tuberculosis invades, leading to misdiagnosis of a primary adrenal tumor or an adrenal metastasis from other organs. Consequently, a single detection method has difficulty distinguishing adrenal TB from malignant metastatic lesions. The complexity of the acquisition of puncture samples also leads to the difficulty of making accurate diagnoses. A histological examination of the specimens is the gold standard for diagnosis. Therefore, it is difficult to diagnose adrenal TB in routine TB-related examinations. As primary adrenal TB is a rare clinical entity, we describe a rare case of primary adrenal TB with a review of the literature on this subject. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-511).
Case presentation
A 59-year-old man was admitted for recurrence of oral (≥0.5 cm) and genital aphthosis (≤0.5 cm) for 3 years, which could not be effectively controlled by irregular medication usage. The patient presented with no family history of adrenal cancer, other malignancy, other endocrinopathy or tuberculosis. Additionally, he had never been vaccinated against tuberculosis and did not come from a tuberculosis endemic region. Chest computed tomography (CT) scan showed multiple small nodules and interstitial changes in both lungs, and enlarged adrenal gland (Figure 1A). The result of sputum cultures for tuberculosis was negative. Based on symptoms of oral and genital aphthosis, the patient was diagnosed with Behcet’s disease according to the International Criteria for Behcet’s disease (8). The laboratory examinations were as follows: the erythrocyte sedimentation rate (ESR) was 20.00 mm/h (normal range: 0–15 mm/h), and the antinuclear antibody (ANA) was positive. The tumor markers [carcinoembryonic antigen (CEA), alpha fetoprotein (AFP), cancer antigen 125 (CA125), carbohydrate antigens], rheumatoid factor, adrenal and pancreatic biochemical evaluations (cortisol, supine and standing aldosterone, serum sodium and potassium, pancreatic amylase and lipase), HLA-B27/HLA-B7 and extractable nuclear antigen (ENA) antibodies were in the normal reference ranges. Then, he was given thalidomide (0.50 g/day, orally) as well as Kangfuxin liquid (10 mL three times a day, orally) to promote aphthosis healing.
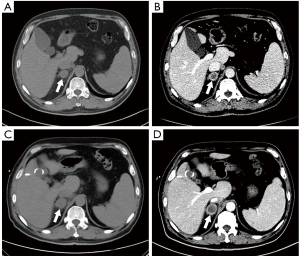
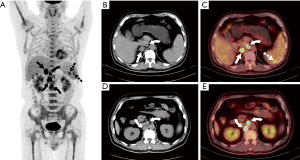
After 10 days of admission, the patient had sudden abdominal pain in the right upper quadrant with a fever of 38.6 °C. Abdominal CT revealed gallstones with a maximum diameter of 0.8 cm, indicating an acute cholecystitis attack. After antibiotic and symptomatic supportive treatment for 3 days, the patient still had intermittent right upper abdominal pain and high fever. As careful preoperative evaluation of the patient’s current general state was performed by anesthesiologists, the risk of complications arising from cholecystectomy was particularly high; therefore, the patient underwent percutaneous transhepatic gallbladder drainage (PTGBD), and his pain relief was significant. However, intermittent fever (up to 39.2 °C) was still present. Blood and bile culture results revealed no pathogenic agents. Further adrenal contrast-enhanced CT showed a right adrenal mass and multiple enlarged retroperitoneal lymph nodes, which exhibited peripheral rim enhancement without calcification (Figure 1B) and indicated that a metastatic adrenal mass was suspected. The dynamic variations in adrenal CT images during hospitalization were present in Figure 1A-1D. PET-CT showed intense 18F-FDG uptake in the right adrenal mass with an SUVmax of 15.2 and foci of intense uptake in the spleen and retroperitoneal lymph nodes (Figure 2). Reconstructed three-dimensional images were presented in Figure 3. A bone marrow puncture only detected leukocytosis without malignant tumor cells.
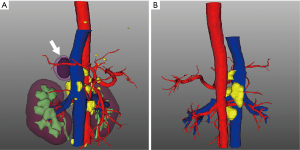
Next, regarding the treatment of the right adrenal mass, considering that percutaneous biopsy might cause the risk of tumor metastasis or dissemination and that the limited number of puncture samples or needles could lead to false-positive or false-negative results, the patient underwent retroperitoneal laparoscopic adrenalectomy. During the operation, enlargement of the right adrenal gland was observed. The fat around the kidney was obviously saponified, and the adhesion of the adrenal mass to the surrounding tissues was severe. The excised mass was 2.2 cm ×2.0 cm ×1.5 cm in size with a medium texture. Intraoperative frozen pathological results showed a higher possibility of tissue-derived inflammatory diseases than the possibility of tumorous diseases. Then, we assessed whether to perform lymph node dissection for the patient. We observed the enlarged retroperitoneal lymph nodes were close to the abdominal aorta, and serious adhesion between the lymph nodes and abdominal aorta was present (Figure 3). Considering the unstable general condition, high risk of bleeding and rupture of the abdominal aorta, increased operation time due to surgical separation of adhesive lymph nodes and the result of intraoperative frozen section analysis, regional lymphadenectomy was not performed after communication with the patient’s family. Histological examination of the specimen revealed primary adrenal TB with a granulomatous lesion containing multinucleated giant cells and inflammatory cells with caseous necrosis. Immunohistochemistry showed CD2 (+), CD3 (+), CD20 (+), CD30 (+), CD163 (+), TIA1 (+), Ki-67 (+), Granzyme (+), CD21 (no FDC network) and CD56 (−). The Epstein-Barr virus-encoded RNA (EBER) test was negative (Figure S1). When given levofloxacin solution (levoflox 500 mg/250 mL, once daily, intravenously) and anti-tuberculosis treatment with isoniazid (0.3 g/day, orally), rifampin (0.45 g/day, orally), pyrazinamide (0.75 g twice a day, orally) and ethambutol tablets (0.75 g/day, orally), the patient’s fever symptom was significantly relieved. He accepted postoperative anti-TB medication for six months. At the 1-year follow-up, no abnormal lumps or abscess recurrence were detected by radiographic examination. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Risk factors for EPTB
Although tuberculosis is often confined to the respiratory system clinically, extrapulmonary dissemination occurs in approximately 15–20% of cases and can lead to devastating clinical consequences (9). EPTB is often misdiagnosed as the clinical symptoms may be obscure and afflict organs at distance from the primary infected site (10). The risk factors for EPTB are receiving increasing attention from researchers and currently include the following broad areas: (I) patient baseline information, including sex and age, may influence the increased risk of developing EPTB. In Morocco, Sbayi et al. analyzed 2,962 EPTB cases from 2000 to 2012 and found males and young populations between 15 and 34 years of age were more likely to be associated with EPTB (11). In the meantime, in Lebanon, O’Son et al. conducted a logistic regression model based on 507 EPTB cases and identified risk factors for EPTB, including female sex and age between 5 and 15 years old (12). In China, a large-scale multicenter observational study found female patients were less inclined to be at higher risk of concurrent PTB, and the risk of concurrent PTB declined with aging (13). (II) The patient’s underlying comorbidities may lead to reduced immune function and an increased incidence of EPTB. Chiu et al. evaluated the association between polycystic kidney disease (PKD) and EPTB in a nationwide population-based cohort study of 13,540 participants. They proposed the PKD cohort demonstrated a significantly higher risk of EPTB than the non-PKD cohort, and medical professionals should preserve a suspicion of EPTB for PKD patients (14). A previous study focused on people living with HIV in Addis Ababa, Ethiopia. Alemu et al. conducted a retrospective study of 566 HIV-positive individuals to investigate the predictors of EPTB. They suggested HIV WHO stage III/IV, CD4 count<50 cells/µL and hemoglobin <10 mg/dL were independent risk factors (15). (III) Some micronutrient deficiencies may be closely linked to the development of EPTB. As vitamin D plays a role in the regulation of immune function by enhancing the antimicrobial activity of monocytes and macrophages, Hammami et al. found vitamin D levels were significantly lower in the EPTB group than in the control group, and vitamin D deficiency was an independent predictor of EPTB (16).
Association between TB and Bechet’s disease
BD is a rare systemic vasculitis with unknown etiology and is characterized by recurrent attacks of oral aphthous ulcers, genital ulcers, ocular lesions and skin lesions (8). The molecular or genetic relationship between TB and BD has attracted the attention of researchers. Efthimiou et al. reported cases of PTB in patients with BD were HLA-B5-positive, which may be a common genetic predisposition. Moreover, BD may be associated with the deficiency of cell-mediated immunity, which contributes to the increased susceptibility to TB (17). Heat shock protein 65 (HSP65) antigens are derived from Mycobacterium, have high homology with HSP60 antigens and are overexpressed in recurrent oral ulcerations, papulopustules and the epidermal areas of erythema nodosum in BD (18), which participates in the molecular biological link between BD and TB. From the perspective of clinical presentation, Mendes et al. proposed “pseudo-BD” can mimic “pure-BD”, presenting with hypersensitivity reactions with M. tuberculosis, including reactive arthritis, erythema nodosum and orogenital ulcers (19). To differentially diagnose “pseudo-BD” related to TB and “pure-BD” without TB, one is to judge whether BD-related symptoms had a complete response after anti-TB therapy (20). In our case, the patient’s BD-related symptoms, including oral and genital lesions, were significantly relieved without recurrence. After the postoperative administration of anti-TB medication for 6 months, the occurrence of “pseudo-BD” may be associated with the pathological effects of TB in the patient. Another method of identification is based on the interferon-γ release assay (IGRA), which may help assess the probability of TB in BD patients (21). After analyzing 173 pure BD patients and 59 BD patients with TB infection, the sensitivity and specificity of IGRA were 88.9% and 74.8%, respectively, and the optimal cutoff for diagnosing active TB was 70/106 spot-forming cells, which improved the diagnostic efficiency of BD patients with active TB (22).
How to discriminate between adrenal TB and other adrenal space-occupying lesions
There are discrepancies between adrenal TB and other adrenal space-occupying lesions in the evolution of disease. At the early stage of TB infection, contrast-enhanced CT imaging reveal enlarged adrenal glands with central necrotic hypodense areas and peripheral enhanced rims. At the late stage, the adrenal gland is atrophied with irregular margins and calcification of the lesion (23). The dynamic changes in adrenal CT images of our patient during hospitalization revealed no calcification or adrenal gland atrophy (Figure 1A-1D). The patient also did not have adrenal insufficiency, which usually occurs when 90% of the gland tissue is destroyed at the late stage of the infection. Therefore, we inferred the patient in our case was at the early phase of adrenal TB infection based on the radiomics signatures. The relevant radiological features among the adrenal infection lesions, malignant adrenal masses and benign adrenal masses are summarized in Table 1. The adrenal gland is a common site for metastases of malignant tumors due to its sufficient blood supply. In general, adrenal TB usually involves both adrenal glands, but the possibility of unilateral involvement cannot be ruled out. In addition, a large, heterogeneous and poorly defined mass with irregular peripheral enhancement can be manifested on contrasted-enhanced CT (71). Calcification and hemorrhagic areas can also sometimes be observed. Unlike the variable contour of TB, most of the primary adrenal tumors appeared as masses or nodules. Both adrenal TB and adrenal metastases can cause gradual destruction of the gland. When 90% of the tissue is destroyed, adrenal cortical dysfunction occurs, which is characterized by Addison’s disease (4). However, the primary adrenal tumor can lead to adrenal hyperfunction instead of adrenocortical insufficiency. A study found less than 10% of adrenal tumors showed calcification (72), but adrenal TB with calcification was seen more frequently (73).
Table 1
| Type of diseases | CT findings | MRI findings | PET-CT findings | 123I-MIBG findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue mass | Density | Necrosis | Calcifications | Hemorrhage | Cystic | T1-weighted (signal) | T2-weighted (signal) | FDG uptake | SUVmax | Uptake | ||||
| Malignant adrenal mass (24-47) | ||||||||||||||
| Cortical carcinoma | Solid | Heterogeneous | + | + | + | − | Low | High | Increased | 7.3 (4–21.8) | – | |||
| Adrenal metastase | Solid | Heterogeneous | + | − | + | − | Low | High | Increased | >3.1 | – | |||
| Neuroblastoma | Solid | Heterogeneous | + | + | + | − | Low | High | Increased | 21 | High dose |
|||
| Lymphoma | Soft | Heterogeneous | +/− | − | − | +/− | Low | High | Increased | 17.24 (9.5–48) | – | |||
| Benign adrenal mass (24-31,48-64) | ||||||||||||||
| Adenoma | Soft | Homogeneous | − | +/− | − | +/- | High | High | Lower | 4.0 | – | |||
| Adrenal cyst | fluid | Homogeneous | − | +/− | +/− | + | Low | High | Lower | – | – | |||
| Myelolipoma (pure fat) | Soft | −30 to −100 HU | − | +/− | − | − | High | High | Lower | 0.7 (0.5-1.1) | – | |||
| Myelolipoma (fat mixed with myeloid) | 1.4 (0.9-2.3) | – | ||||||||||||
| Hemangioma (early) | Soft | Heterogeneous | − | − | + | +/− | Low | High | – | – | – | |||
| Hemangioma (late) | + | + | ||||||||||||
| Ganglioneuroma | Solid | Homogeneous | − | +/− | − | − | High | Low/equivalent/ high | Lower | 2.4 (1.5–2.9) | – | |||
| Schwannoma | Soft | Heterogeneous | − | + | + | + | Equivalent | Slightly high | Increased | 9.5 | – | |||
| Pheocromocytoma | Solid | Heterogeneous | + | + | +/− | + | Low | Equivalent/high | Increased | – | High dose |
|||
| Adrenal Infection (23,65-70) | ||||||||||||||
| Tuberculosis | Soft | Heterogeneous | +/− | + | +/− | + | Low/equivalent | High | Increased | 15.2 | – | |||
| Histoplasmosis | Soft | Heterogeneous | + | + | + | +/− | Low | Equivalent | Increased | 19.7–24.5 | – | |||
How to discriminate between primary and secondary adrenal TB
Secondary adrenal TB usually occurs bilaterally, and some studies found adrenal calcification is the main imaging feature of secondary adrenal TB (74). Morgan et al. reported adrenal TB, secondary to PTB, had bilateral adrenal calcification, while renal computed tomography showed bilateral adrenal mass-like enlargement (75). The main reason is that the hematogenous or lymphoid transmission of Mycobacterium tuberculosis infection has the same effect on both adrenal glands (74). After searching the relevant electronic literature, primary adrenal TB may be suspected of being diagnosed when it has the following characteristics (3,76): (I) the clinical presentation includes fever, chills, night sweats and weight loss, (II) the laboratory findings include positive IgG anti-TB antibodies, positive purified protein derivative (PPD) test and high ESR, (III) the radiological results of adrenal involvement include CT, MRI and PET-CT, (IV) no other imaging or clinical signs of extra-adrenal TB are observed, and (V) the patient has no prior or current history of tuberculosis. If adrenal TB was not isolated occurrence in any part of the body or if the patient had adrenal TB secondary to primary tuberculosis in the respiratory system, digestive system, urinary system, etc., secondary adrenal TB was highly suspected. Furthermore, characteristics of reported cases of primary adrenal TB were summarized in Table 2.
Table 2
| First author (year) | Age at diagnosis (sex) | Presenting symptoms | CT | Calcification | Addison’s disease | Treatment | Follow-up (months) | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Adrenal mass size (cm) | Bilateral/unilateral | Anti-TB therapy | Cortisol replacement therapy | |||||||
| Wan et al., 2021 (5) | 46 (F) | Right lumbar pain, weight loss and fatigue | Left: 3.1×2.1 Right: 3.5×2.5 | Bilateral | No | No | Isoniazid and rifampin | No | NR | NR |
| 29 (F) | Back pain, fever and sweating | Right: 3.4×2.3 | Unilateral (right) | No | No | Isoniazid and rifampin | No | NR | NR | |
| Abdalla et al., 2021 (77) | 42 (M) | Weight loss and fatigue | Normal | Unilateral (left) | Yes | Yes | No | Hydrocortisone | 3 | Improvement |
| Yu et al., 2020 (78) | 51 (F) | Skin pigmentation | NR | Bilateral | No | Yes | Isoniazid and rifampin | Hydrocortisone | 15 | Improvement |
| Arambewela et al., 2019 (79) | 78 (F) | NR | Left: 2.0×2.0 Right: 1.6×1.6 | Bilateral | No | Yes | Specific unknown | Hydrocortisone | NR | Improvement |
| Soedarso et al., 2018 (80) | 43 (M) | Skin pigmentation, weight loss, fatigue, nausea and anorexia | NR | Bilateral | No | Yes | Specific unknown | Hydrocortisone | NR | Improvement |
| Jang et al., 2017 (81) | 66 (F) | Skin pigmentation, weight loss, nausea, vomiting | NR | Bilateral | No | Yes | Specific unknown | Hydrocortisone | NR | NR |
| Upadhyay et al., 2014 (82) | 46 (M) | Weight loss, fatigue and anorexia | NR | Bilateral | No | Yes | QAC | Hydrocortisone | NR | Improvement |
| Shrestha et al., 2013 (76) | 36 (M) | Skin pigmentation and fatigue | NR | Bilateral | No | Yes | QAC | Prednisolone | 27 | Improvement |
| Del Borgo et al., 2010 (83) | 61 (M) | Fatigue, fever and night sweats | Left: 6.0×6.0 Right: 6.0×6.0 | Bilateral | No | No | Isoniazid , rifampicin, pyrazinamide, ethambutol and streptomycin | No | 9 | Improvement |
| Liatsikos et al., 2006 (84) | 25 (M) | Weight loss, fatigue and anorexia | NR | Bilateral | No | Yes | QAC | Hydrocortisone | 1 | Improvement |
| Ikoma et al., 2004 (85) | 68 (M) | Persistent intermittent fever | 2.8×2.0 | Unilateral (Right) | No | No | Isoniazid | Not used | 3 | Improvement |
| Serter et al., 2003 (86) | 61 (M) | Skin pigmentation, weight loss, fatigue, nausea, vomiting, anorexia and diarrhea | Left: NR Right: 6×3.5 | Bilateral | No | Yes | QAC | Prednisolone andfludrocortisone | NR | NR |
| Llewelyn et al., 1999 (87) | 56 (M) | Skin pigmentation and lethargy | NR | Bilateral | Yes | Yes | QAC | Hydrocortisone | NR | Improvement |
| Ward et al., 1985 (88) | 65 (M) | Weight loss | NR | Bilateral | NR | NR | NR | NR | NR | Death |
| Our case | 59 (M) | Recurrent oral ulcers | 3.1×2.5 | Unilateral (right) | No | No | QAC | No | 14 | Improvement |
F, female; M, male; NR, not reported; QAC, quadruple antituberculous chemotherapy (Isoniazid, rifampicin, pyrazinamide, and ethambutol).
Conclusions
Primary unilateral adrenal TB without adrenal insufficiency is a rare type of EPTB. 18FDG PET/CT scans have limitations in differentiating rare metastatic tumors and TB. Due to the relatively low diagnostic efficiency of sputum smear microscopy, rapid molecular tests, culture-based methods and imaging approaches, adrenalectomy or percutaneous biopsy may be required for the diagnosis of adrenal TB, especially without evidence of extra-adrenal disease.
Acknowledgments
The authors would like to acknowledge Three-dimensional visualization technology, YORKTAL.
Funding: The present study was supported by grants from the National Natural Science Foundation of China (grant No. 31800787), the Natural Science Foundation of Liaoning Province (grant No. LQ2017025), the Doctoral Research Startup Foundation of Liaoning Province (grant No. 20180540020), the Medical Scientific Research Project of Dalian City (grant No. 1812038) and the United Fund of the Second Hospital of Dalian Medical University and Dalian Institute of Chemical Physics, Chinese Academy of Sciences (UF-QN-202004).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-511
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-511). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lam KY, Lo CY. A critical examination of adrenal tuberculosis and a 28-year autopsy experience of active tuberculosis. Clin Endocrinol (Oxf) 2001;54:633-9. [Crossref] [PubMed]
- Addison T. A Collection of the published writings of the late Thomas Addison, MD. 1868.
- Løvås K, Husebye ES. Addison's disease. Lancet 2005;365:2058-61. [Crossref] [PubMed]
- Kelestimur F. The endocrinology of adrenal tuberculosis: the effects of tuberculosis on the hypothalamo-pituitary-adrenal axis and adrenocortical function. J Endocrinol Invest 2004;27:380-6. [Crossref] [PubMed]
- Wan S, Du F, Wang J, et al. Primary unilateral and epilepsy adrenal tuberculosis misdiagnosed as adrenal tumor: Report of two cases. Asian J Surg 2021;44:1461-3. [Crossref] [PubMed]
- Furin J, Cox H, Pai M. Tuberculosis. Lancet 2019;393:1642-56. [Crossref] [PubMed]
- van Haren Noman S, Visser H, Muller AF, et al. Addison's Disease Caused by Tuberculosis: Diagnostic and Therapeutic Difficulties. Eur J Case Rep Intern Med 2018;5:000911. [Crossref] [PubMed]
- International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD). The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014;28:338-47. [Crossref] [PubMed]
- Krishnan N, Robertson BD, Thwaites G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2010;90:361-6. [Crossref] [PubMed]
- Solovic I, Jonsson J, Korzeniewska-Koseła M, et al. Challenges in diagnosing extrapulmonary tuberculosis in the European Union, 2011. Euro Surveill 2013; [Crossref] [PubMed]
- Sbayi A, Arfaoui A, Janah H, et al. Epidemiological characteristics and some risk factors of extrapulmonary tuberculosis in Larache, Morocco. Pan Afr Med J 2020;36:381. [Crossref] [PubMed]
- O'Son L, Hulland E, Cookson ST, et al. Epidemiology and risk factors for extrapulmonary tuberculosis in Lebanon. Int J Tuberc Lung Dis 2020;24:414-9. [Crossref] [PubMed]
- Kang W, Yu J, Du J, et al. The epidemiology of extrapulmonary tuberculosis in China: A large-scale multi-center observational study. PLoS One 2020;15:e0237753. [Crossref] [PubMed]
- Chiu TF, Yu TM, Chiu CW, et al. Increased risk of pulmonary and extrapulmonary tuberculosis infection in patients with polycystic kidney disease: a nationwide population-based study with propensity score-matching analysis. J Transl Med 2021;19:253. [Crossref] [PubMed]
- Alemu A, Yesuf A, Gebrehanna E, et al. Incidence and predictors of extrapulmonary tuberculosis among people living with Human Immunodeficiency Virus in Addis Ababa, Ethiopia: A retrospective cohort study. PLoS One 2020;15:e0232426. [Crossref] [PubMed]
- Hammami F, Koubaa M, Mejdoub Y, et al. The association between vitamin D deficiency and extrapulmonary tuberculosis: Case-control study. Tuberculosis (Edinb) 2021;126:102034. [Crossref] [PubMed]
- Efthimiou J, Hay PE, Spiro SG, et al. Pulmonary tuberculosis in Behçet's syndrome. Br J Dis Chest 1988;82:300-4. [Crossref] [PubMed]
- Direskeneli H. Innate and Adaptive Responses to Heat Shock Proteins in Behcet's Disease. Genet Res Int 2013;2013:249157. [Crossref] [PubMed]
- Mendes D, Correia M, Barbedo M, et al. Behçet's disease--a contemporary review. J Autoimmun 2009;32:178-88. [Crossref] [PubMed]
- Shinoda K, Hayashi R, Taki H, et al. Pseudo-Behçet's disease associated with tuberculosis: a case report and review of the literature. Rheumatol Int 2014;34:1471-4. [Crossref] [PubMed]
- Cho S, Lee KJ, Lee JD, et al. Detection of tuberculous lymphadenopathy by positron emission tomography/computed tomography in a patient with Behçet's disease. Acta Derm Venereol 2011;91:470-1. [Crossref] [PubMed]
- Wu X, Chen P, Wei W, et al. Diagnostic value of the interferon-γ release assay for tuberculosis infection in patients with Behçet's disease. BMC Infect Dis 2019;19:323. [Crossref] [PubMed]
- Buxi TB, Vohra RB. CT in adrenal enlargement due to tuberculosis: a review of literature with five new cases. Clin Imaging 1992;16:102-8. [Crossref] [PubMed]
- Reginelli A, Vacca G, Belfiore M, et al. Pitfalls and differential diagnosis on adrenal lesions: current concepts in CT/MR imaging: a narrative review. Gland Surg 2020;9:2331-42. [Crossref] [PubMed]
- Kandathil A, Wong KK, Wale DJ, et al. Metabolic and anatomic characteristics of benign and malignant adrenal masses on positron emission tomography/computed tomography: a review of literature. Endocrine 2015;49:6-26. [Crossref] [PubMed]
- d'Amuri FV, Maestroni U, Pagnini F, et al. Magnetic resonance imaging of adrenal gland: state of the art. Gland Surg 2019;8:S223-32. [Crossref] [PubMed]
- He X, Caoili EM, Avram AM, et al. 18F-FDG-PET/CT Evaluation of Indeterminate Adrenal Masses in Noncancer Patients. J Clin Endocrinol Metab 2021;106:1448-59. [Crossref] [PubMed]
- Sundin A, Hindié E, Avram AM, et al. A Clinical Challenge: Endocrine and Imaging Investigations of Adrenal Masses. J Nucl Med 2021;62:26S-33S. [Crossref] [PubMed]
- Wang F, Liu J, Zhang R, et al. CT and MRI of adrenal gland pathologies. Quant Imaging Med Surg 2018;8:853-75. [Crossref] [PubMed]
- Schieda N, Siegelman ES. Update on CT and MRI of Adrenal Nodules. AJR Am J Roentgenol 2017;208:1206-17. [Crossref] [PubMed]
- Schieda N, Alrashed A, Flood TA, et al. Comparison of Quantitative MRI and CT Washout Analysis for Differentiation of Adrenal Pheochromocytoma From Adrenal Adenoma. AJR Am J Roentgenol 2016;206:1141-8. [Crossref] [PubMed]
- Ishiwata K, Suzuki S, Igarashi K, et al. Characteristics of benign adrenocortical adenomas with 18F-FDG PET accumulation. Eur J Endocrinol 2021;185:155-65. [Crossref] [PubMed]
- Ahmed AA, Thomas AJ, Ganeshan DM, et al. Adrenal cortical carcinoma: pathology, genomics, prognosis, imaging features, and mimics with impact on management. Abdom Radiol (NY) 2020;45:945-63. [Crossref] [PubMed]
- Wong KK, Miller BS, Viglianti BL, et al. Molecular Imaging in the Management of Adrenocortical Cancer: A Systematic Review. Clin Nucl Med 2016;41:e368-82. [Crossref] [PubMed]
- Fintelmann FJ, Tuncali K, Puchner S, et al. Catecholamine Surge during Image-Guided Ablation of Adrenal Gland Metastases: Predictors, Consequences, and Recommendations for Management. J Vasc Interv Radiol 2016;27:395-402. [Crossref] [PubMed]
- Launay N, Silvera S, Tenenbaum F, et al. Value of 18-F-FDG PET/CT and CT in the Diagnosis of Indeterminate Adrenal Masses. Int J Endocrinol 2015;2015:213875. [Crossref] [PubMed]
- Wagnerova H, Lazurova I, Felsoci M. Adrenal metastases. Bratisl Lek Listy 2013;114:237-40. [PubMed]
- Albano D, Agnello F, Midiri F, et al. Imaging features of adrenal masses. Insights Imaging 2019;10:1. [Crossref] [PubMed]
- Kushner BH. Neuroblastoma: a disease requiring a multitude of imaging studies. J Nucl Med 2004;45:1172-88. [PubMed]
- Rha SE, Byun JY, Jung SE, et al. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics 2003;23:29-43. [Crossref] [PubMed]
- Sofka CM, Semelka RC, Kelekis NL, et al. Magnetic resonance imaging of neuroblastoma using current techniques. Magn Reson Imaging 1999;17:193-8. [Crossref] [PubMed]
- Tateishi U, Hasegawa T, Makimoto A, et al. Adult neuroblastoma: radiologic and clinicopathologic features. J Comput Assist Tomogr 2003;27:321-6. [Crossref] [PubMed]
- Laurent C, Casasnovas O, Martin L, et al. Adrenal lymphoma: presentation, management and prognosis. QJM 2017;110:103-9. [PubMed]
- Zhou L, Peng W, Wang C, et al. Primary adrenal lymphoma: radiological; pathological, clinical correlation. Eur J Radiol 2012;81:401-5. [Crossref] [PubMed]
- Zhang LJ, Yang GF, Shen W, et al. Imaging of primary adrenal lymphoma: case report and literature review. Acta Radiol 2006;47:993-7. [Crossref] [PubMed]
- Kumar R, Xiu Y, Mavi A, et al. FDG-PET imaging in primary bilateral adrenal lymphoma: a case report and review of the literature. Clin Nucl Med 2005;30:222-30. [Crossref] [PubMed]
- Skoura E, Oikonomopoulos G, Vasileiou S, et al. (18)F-FDG-PET/CT, (123)I-MIBG and (99m)Tc-MDP whole-body scans, in detecting recurrence of an adult adrenal neuroblastoma. Hell J Nucl Med 2014;17:58-61. [PubMed]
- Yamamoto T, Koizumi M, Kohno A, et al. A case report on 111In chloride bone marrow scintigraphy in management of adrenal myelolipoma. Medicine (Baltimore) 2019;98:e14625. [Crossref] [PubMed]
- Littrell LA, Carter JM, Broski SM, et al. Extra-adrenal myelolipoma and extramedullary hematopoiesis: Imaging features of two similar benign fat-containing presacral masses that may mimic liposarcoma. Eur J Radiol 2017;93:185-94. [Crossref] [PubMed]
- Geng C, Liu N, Yang G, et al. Primary mediastinal myelolipoma: A case report and review of the literature. Oncol Lett 2013;5:862-4. [Crossref] [PubMed]
- Ioannidis O, Papaemmanouil S, Chatzopoulos S, et al. Giant bilateral symptomatic adrenal myelolipomas associated with congenital adrenal hyperplasia. Pathol Oncol Res 2011;17:775-8. [Crossref] [PubMed]
- Wilson B, Becker A, Estes T, et al. Adrenal Hemangioma Definite Diagnosis on CT, MRI, and FDG PET in a Patient With Primary Lung Cancer. Clin Nucl Med 2018;43:e192-4. [Crossref] [PubMed]
- Calata JF, Sukerkar AN, August CZ, et al. Benign adrenal hemangiomas may mimic metastases on PET. Clin Nucl Med 2013;38:888-90. [Crossref] [PubMed]
- Marotti M, Sucić Z, Krolo I, et al. Adrenal cavernous hemangioma: MRI, CT, and US appearance. Eur Radiol 1997;7:691-4. [Crossref] [PubMed]
- Shawa H, Elsayes KM, Javadi S, et al. Adrenal ganglioneuroma: features and outcomes of 27 cases at a referral cancer centre. Clin Endocrinol (Oxf) 2014;80:342-7. [Crossref] [PubMed]
- Linos D, Tsirlis T, Kapralou A, et al. Adrenal ganglioneuromas: incidentalomas with misleading clinical and imaging features. Surgery 2011;149:99-105. [Crossref] [PubMed]
- Shao M, Zhang W, Niu Z, et al. Computed tomography characteristics of adrenal ganglioneuroma: a retrospective analysis of 30 pathologically-confirmed cases. J Int Med Res 2020;48:300060520945510. [Crossref] [PubMed]
- Majbar AM, Elmouhadi S, Elaloui M, et al. Imaging features of adrenal ganglioneuroma: a case report. BMC Res Notes 2014;7:791. [Crossref] [PubMed]
- Liu QY, Gao M, Li HG, et al. Juxta-adrenal schwannoma: dynamic multi-slice CT and MRI findings. Eur J Radiol 2012;81:794-9. [Crossref] [PubMed]
- Adas M, Ozulker F, Adas G, et al. A rare adrenal incidentaloma: adrenal schwannoma. Case Rep Gastroenterol 2013;7:420-7. [Crossref] [PubMed]
- Tang W, Yu XR, Zhou LP, et al. Adrenal schwannoma: CT, MR manifestations and pathological correlation. Clin Hemorheol Microcirc 2018;68:401-12. [Crossref] [PubMed]
- Sbardella E, Grossman AB. Pheochromocytoma: An approach to diagnosis. Best Pract Res Clin Endocrinol Metab 2020;34:101346. [Crossref] [PubMed]
- Vyakaranam AR, Crona J, Norlén O, et al. 11C-hydroxy-ephedrine-PET/CT in the Diagnosis of Pheochromocytoma and Paraganglioma. Cancers (Basel) 2019;11:847. [Crossref] [PubMed]
- Hescot S, Curras-Freixes M, Deutschbein T, et al. Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J Clin Endocrinol Metab 2019;104:2367-74. [Crossref] [PubMed]
- Roudaut N, Malecot JM, Dupont E, et al. Adrenal tuberculosis revealed by FDG PET. Clin Nucl Med 2008;33:821-3. [Crossref] [PubMed]
- Li YJ, Cai L, Sun HR, et al. Increased FDG uptake in bilateral adrenal tuberculosis appearing like malignancy. Clin Nucl Med 2008;33:191-2. [Crossref] [PubMed]
- Zhang XC, Yang ZG, Li Y, et al. Addison's disease due to adrenal tuberculosis: MRI features. Abdom Imaging 2008;33:689-94. [Crossref] [PubMed]
- Narang V, Sinha T, Sandhu AS, et al. Clinically inapparent bilateral adrenal masses due to histoplasmosis. Eur Urol 2009;55:518-21. [Crossref] [PubMed]
- Kumar N, Singh S, Govil S. Adrenal histoplasmosis: clinical presentation and imaging features in nine cases. Abdom Imaging 2003;28:703-8. [Crossref] [PubMed]
- Sanders V, Donald J, Siegel BA. Adrenal Histoplasmosis. Clin Nucl Med 2018;43:502-3. [Crossref] [PubMed]
- Lockhart ME, Smith JK, Kenney PJ. Imaging of adrenal masses. Eur J Radiol 2002;41:95-112. [Crossref] [PubMed]
- Kenney PJ, Stanley RJ. Calcified adrenal masses. Urol Radiol 1987;9:9-15. [Crossref] [PubMed]
- Wang YX, Chen CR, He GX, et al. CT findings of adrenal glands in patients with tuberculous Addison's disease. J Belge Radiol 1998;81:226-8. [PubMed]
- Yang ZG, Guo YK, Li Y, et al. Differentiation between tuberculosis and primary tumors in the adrenal gland: evaluation with contrast-enhanced CT. Eur Radiol 2006;16:2031-6. [Crossref] [PubMed]
- Morgan HE, Austin JH, Follet DA. Bilateral adrenal enlargement in Addison's disease caused by tuberculosis. Nephrotomographic demonstration. Radiology 1975;115:357-8. [Crossref] [PubMed]
- Shrestha B, Omran A, Rong P, et al. Successfully treated unusual case of primary adrenal and spinal tuberculosis with three years follow up. Pan Afr Med J 2014;17:108. [Crossref] [PubMed]
- Abdalla M, Dave JA, Ross IL. Addison's disease associated with hypokalemia: a case report. J Med Case Rep 2021;15:131. [Crossref] [PubMed]
- Yu J, Lu Y, Han B. Primary adrenal insufficiency due to adrenal tuberculosis: a case report. J Int Med Res 2020;48:300060520980590. [Crossref] [PubMed]
- Arambewela M, Ross R, Pirzada O, et al. Tuberculosis as a differential for bilateral adrenal masses in the UK. BMJ Case Rep 2019;12:228532. [Crossref] [PubMed]
- Soedarso MA, Nugroho KH, Meira Dewi KA. A case report: Addison disease caused by adrenal tuberculosis. Urol Case Rep 2018;20:12-4. [Crossref] [PubMed]
- Jang SA, Park JH, Lee KA. Primary adrenal and chest wall tuberculosis presenting as an adrenal crisis. QJM 2017;110:389-90. [Crossref] [PubMed]
- Upadhyay J, Sudhindra P, Abraham G, et al. Tuberculosis of the adrenal gland: a case report and review of the literature of infections of the adrenal gland. Int J Endocrinol 2014;2014:876037. [Crossref] [PubMed]
- Del Borgo C, Urigo C, Marocco R, et al. Diagnostic and therapeutic approach in a rare case of primary bilateral adrenal tuberculosis. J Med Microbiol 2010;59:1527-9. [Crossref] [PubMed]
- Liatsikos EN, Kalogeropoulou CP, Papathanassiou Z, et al. Primary adrenal tuberculosis: role of computed tomography and CT-guided biopsy in diagnosis. Urol Int 2006;76:285-7. [Crossref] [PubMed]
- Ikoma A, Namai K, Saito T, et al. Unilateral active adrenal tuberculosis featuring persistent intermittent fever. Endocr J 2004;51:463-6. [Crossref] [PubMed]
- Serter R, Koç G, Demirbas B, et al. Acute adrenal crisis together with unilateral adrenal mass caused by isolated tuberculosis of adrenal gland. Endocr Pract 2003;9:157-61. [Crossref] [PubMed]
- Llewelyn M, Adler M, Steer K, et al. Acute adrenal insufficiency precipitated by isolated involvement of the adrenal gland by tuberculosis. J Infect 1999;39:244-5. [Crossref] [PubMed]
- Ward S, Evans CC. Sudden death due to isolated adrenal tuberculosis. Postgrad Med J 1985;61:635-6. [Crossref] [PubMed]


