
Use of the peritoneum or the round ligament of the liver in radical surgery for pancreatic cancer
Introduction
Pancreatic cancer is a common gastrointestinal tumor. Due to its insidious onset and atypical early symptoms, pancreatic cancer is most often detected at middle and late stages. In addition, due to the special anatomical relationships between the pancreas and the portal vein (PV)/superior mesenteric vein (SMV), pancreatic cancer is prone to invade the PV and SMV (1). When pancreatic cancer involves the PV, SMV, and splenic vein, pancreaticoduodenectomy often requires resection of the involved veins and subsequent venous reconstruction. The ideal graft for venous reconstruction is still unclear. The materials for venous reconstruction are usually artificial blood vessels or autologous veins. Artificial blood vessels are expensive and require postoperative anticoagulation (2). Autologous vein extraction can increase the duration of surgery and the incidence of postoperative infection and can even affect the function of the organs from which the autologous veins are taken, causing lower extremity edema or physical injury (3,4). Our team had also used allogeneic blood vessels for venous reconstruction, but they were difficult to obtain, and required precise storage conditions. In addition, although examinations such as computed tomography (CT) and magnetic resonance imaging (MRI) are completed before surgery for patients with pancreatic cancer, some cases of invasion of the PV, SMV, and splenic vein still found incidentally during surgery.
Thus, the use of peritoneum or the round ligament of the liver for venous reconstruction is not only convenient, rapidly available, and immediately transplantable but also requires no postoperative anticoagulation. Therefore, it is a good material for venous reconstruction. Some studies have cut the hepatic round ligament, remove the surface adipose tissue, reveal the umbilical cord vein cable, find the potential cava of the umbilical vein cord, and reconstruct the corresponding length with the biliary dilation probe. However, our study is to remove the appropriate size of the peritoneal or hepatic round ligament, remove part of the adipose tissue, and use the peritoneal surface of the smooth peritoneal surface or the hepatic round ligament as the inner wall. This study aimed to retrospectively analyze the data from the 11 patients with pancreatic cancer who underwent surgery combined with resection and reconstruction of the PV and SMV using the peritoneum or the round ligament of the liver at the Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, and the Department of General Surgery, Affiliated Hospital of Shaoxing University. All 11 patients completed the operation successfully, including 5 had complications: 3 pancreatic leakage, 1 bleeding and 1 thrombosis; one patient recovered after secondary surgery, and the other patients recovered after conservative treatment.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-712).
Methods
Patients
Data were collected from 11 patients who underwent pancreatic cancer surgery combined with resection and reconstruction of the PV and SMV using the peritoneum (including the round ligament of the liver) from November 2018 to November 2020 at the Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Zhejiang University School of Medicine, and the Department of General Surgery, Affiliated Hospital of Shaoxing University. The patients comprised 5 males and 6 females with a median age of 62 years and an age range of 48–72 years.
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Human Research Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (No. 2021 0939) and individual consent for this retrospective analysis was waived.
Inclusion and exclusion criteria
Inclusion criteria included the following: (I) age 18–80 years; (II) preoperative imaging examination suggesting the presence of pancreatic cancer; (III) preoperative assessment showing the absence of invasion or distant metastasis to the celiac trunk and/or superior mesenteric artery (SMA); (IV) presence of resectable or potentially resectable pancreatic cancer on preoperative assessment according to the 2015 National Comprehensive Cancer Network (NCCN) Guidelines; (V) preoperative assessment showing the absence of contraindications for surgery; (VI) half or less of the circumferences of the PV and SMV are invaded.
Exclusion criteria included the following: (I) preoperative comprehensive assessment showing the presence of distant metastases of pancreatic cancer; (II) presence of unresectable pancreatic cancer on preoperative assessment according to the 2015 NCCN Guidelines; (III) preoperative assessment showing an inability to tolerate surgery; (IV) preoperative assessment showing the presence of invasion of or distant metastasis to the celiac trunk and/or SMA; (V) the patient or his/her family refuses surgical treatment; (VI) more than half of the circumferences of the PV and SMV are invaded.
Surgical method
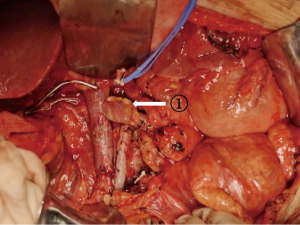
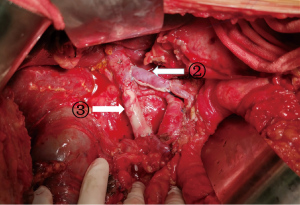
Pancreaticoduodenectomy (excluding blood vessels invaded by tumors) or total pancreatectomy (TP) (excluding blood vessels invaded by tumors) + splenectomy were performed; peripheral lymph node resection involved the anterior and posterior pancreaticoduodenal lymph nodes, the lymph nodes on the right side of the SMA, the hepatoduodenal ligament, the lymph nodes in the upper and lower parts of the head/body of the pancreas, and the retroperitoneal lymph nodes. After the above steps were completed, only the connection between the pancreas and the PV/SMV was preserved. At the same time, we confirmed that <1/2 of the circumferences of the PV and SMV are invaded. According to the area covered by tumor invasion in the veins, an appropriately size tissue segment (i.e., autologous graft material) was removed from the peritoneum or the round ligament of the liver with tissue scissors, remove the appropriate adipose tissue and washed with heparinized saline. The proximal PV and the distal mesenteric vein were clamped with vascular clips, and the time to venous blockage was monitored. The vein invaded by tumors was resected, the specimen was removed, and the venous margin and the smooth surface of the peritoneum (Figure 1: ① splenic vein for peritoneal reconstruction) or the peritoneal surface of the round ligament of the liver (Figure 2: ② round ligament of the liver for the reconstruction of the splenic vein, and ③ round ligament of the liver for the reconstruction of the SMV) were continuously sutured with 5-0 or 6-0 protein stitches to complete venous reconstruction.
Observation index
The surgical procedure, duration of surgery, volume of intraoperative bleeding, vascular invasion status, TNM stage, and periprocedural complications were observed.
Statistical analysis
Data were analyzed using SPSS 19.0. Normally distributed measurement data are expressed as the mean ± SD, whereas nonnormally distributed measurement data are expressed as the median (range); Count data are expressed as absolute numbers.
Results
All 11 patients underwent successful vascular resection of the portal venous system (PVS) and vascular reconstruction with the peritoneum or the round ligament of the liver [Figure 3: ④ CT images of the portal vein with preoperative tumor invasion; Figure 4: ⑤ CT images of the reconstructed portal vein]. The procedures included TP in 4 patients and pancreatoduodenectomy (PD) in 7 patients. For the 11 patients, the duration of surgery was 503±183 min, and the volume of intraoperative bleeding was 332±268 mL. The vascular invasion status, TNM stage, materials used for vascular reconstruction, and length of the reconstructed blood vessel are shown in Table 1. Postoperative pathological examination showed that 10 patients had adenocarcinoma, and 1 patient had a neuroendocrine tumor. Postoperative complications included pancreatic leak in 3 patients, bleeding in 1 patient (after graft necrosis due to excessive thickness of the graft-peritoneal surface fat), and thrombosis in 1 patient (in the reconstructed blood vessel due to postoperative peritonitis). Of these patients, 1 recovered after secondary surgery, and the remaining patients were cured after conservative treatment.
Table 1
| Case | Age (years) | Disease | TMN stage | Procedure | Vascular invasion | Material for reconstruction | Reconstructed length (cm) | Duration of surgery (min) | Intraoperative blood loss (mL) | Surgical complication |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | Adenocarcinoma | IIB | TP | PV-SMV | LTH | 70 | 320 | 200 | Absent |
| 2 | 52 | Adenocarcinoma | IIA | TP | PV-SMV | LTH | 90 | 340 | 150 | Absent |
| 3 | 72 | Neuroendocrine tumor | IIB | TP | SMV | LTH | 60 | 390 | 300 | Absent |
| 4 | 63 | Adenocarcinoma | IIB | TP | PV | LTH | 60 | 440 | 200 | Pancreatic leak |
| 5 | 69 | Adenocarcinoma | IIA | PD | PV-SV | LTH + peritoneum | 70 | 630 | 450 | Absent |
| 6 | 64 | Adenocarcinoma | IIA | PD | PV-SV | LTH | 75 | 605 | 500 | Absent |
| 7 | 48 | Adenocarcinoma | IIB | PD | PV | LTH | 55 | 600 | 400 | Bleeding |
| 8 | 73 | Adenocarcinoma | IIB | PD | SV-SMV | LTH + peritoneum | 50 | 580 | 600 | Pancreatic leak |
| 9 | 56 | Adenocarcinoma | IIB | PD | PV-SMV | LTH | 75 | 610 | 200 | Absent |
| 10 | 60 | Adenocarcinoma | IIB | PD | PV | LTH | 50 | 550 | 350 | Thrombosis |
| 11 | 65 | Adenocarcinoma | IIB | PD | PV | LTH | 60 | 470 | 300 | Pancreatic leak |
PD, pancreatoduodenectomy; TP, total pancreatectomy; PV, portal vein; SMV, superior mesenteric vein; SV, splenic vein; LTH, ligamentum teres hepatis.
Discussion
Only 10–20% of patients have resectable pancreatic cancer at presentation (5). The main reason for this low proportion is that the cancer has invaded major blood vessels, such as the PV and SMV. Invasion can present as infiltration or chronic inflammation (6). If the PV is invaded by tumors, combined vascular resection is an effective treatment that improves the resection rate and the radical surgery rate for pancreatic cancer (6,7). Numerous retrospective studies have shown that in patients undergoing pancreaticoduodenectomy combined with PV-SMV resection, survival is not statistically significantly different from that of patients undergoing standard pancreaticoduodenectomy (8-10). Nonetheless, resection of invaded blood vessels and vascular reconstruction increase the resection rate for pancreatic cancer (6,7,11) and are conducive to patient prognosis. Therefore, vascular reconstruction is worthwhile.
Materials commonly used for PV/SMV reconstruction are derived from the following three sources: artificial blood vessels (12,13), autologous blood vessels (such as the great saphenous vein, left renal vein, umbilical vein, internal jugular vein, etc.) (13-15), and allogeneic blood vessels (i.e., cadaveric blood vessels, such as the PV, etc.) (15,16).
Artificial blood vessels are well tolerated after transplantation. Their appropriate size and length can be determined by to the patient’s condition. However, they are expensive and have poor biocompatibility and low long-term patency. After transplantation with artificial blood vessels, it is necessary to initiate the use of anticoagulants immediately, which increases the risk of abdominal bleeding.
Autologous venous graft has good biocompatibility and high long-term patency rate, but poor caliber matching with replacement vessels and limited length. In addition, it is difficult to take materials, have some damage to the body, and even sacrifice some function of the organs. The great saphenous vein is commonly used for autologous blood vessel transplantation. It allows convenient harvesting of a sufficient length, offers good biocompatibility, and does not require immunosuppressive treatment after surgery. However, its diameter is smaller than that of the PV and SMV, and it is more difficult to obtain than the peritoneum and the round ligament of the liver, which results in some damage to the body during harvesting. Use of the renal vein requires the sacrifice of some organ functions.
Cadaveric venous allografts with an appropriate length and width have advantages and can be implanted in a “Y” shape. However, these blood vessels are obtained from dead bodies and need to be macroscopically evaluated; furthermore, they must be stored under precise conditions for preservation after size determination. In addition, the use of cadaveric allografts requires postoperative immunosuppressive therapy, which increases the risk of tumor recurrence.
The peritoneum and the round ligament of the liver are normal autologous tissues whose resection does not damage functional blood vessels. Their wall thickness is similar to that of the vein wall, and anastomosis is easy to perform; furthermore, such autologous tissues require no anticoagulant therapy after surgery and can be harvested conveniently and rapidly during abdominal surgery. When PV/SMV resection is required, especially when tumor invasion of a vein and the need for venous reconstruction is found incidentally during surgery, an autologous peritoneum or round ligament of the liver can be considered for vein reconstruction.
The round ligament of the liver is formed by atresia of the left umbilical vein during the embryonic period. Its histological structure is similar to that of the large abdominal vein, its wall is characterized by increasing stress/tension and expandability, and its inner surface is visibly covered by intact endothelial cells, which not only provide a cytological basis for the smooth inner surface of the round ligament of the liver but also secrete many biologically active substances that can reduce the likelihood of thrombosis (17). In the past, when the PV system of patients with pancreatic cancer was invaded, an appropriate length of the round ligament of the liver was often selected during surgery and recanalized using certain techniques to repair and reconstruct the PV and SMV.
In our study, a section of the round ligament of the liver with an appropriate length and width was selected, excess adipose tissue was cut off, and the peritoneal surface was anastomosed to the PV or SMV to repair and reconstruct the PV system. In the study, 1 patient experienced postoperative bleeding after graft necrosis due to excessive thickness of the graft-peritoneal surface fat; therefore, excess fatty tissue was cut off to reduce the likelihood of postoperative necrosis. We believe that during surgery, the peritoneum and the round ligament of the liver should be trimmed neatly, and excess adipose tissue should be cut off; the peritoneal surface of the round ligament of the liver and the smooth surface of the peritoneum should oppose the venous endometrium during anastomosis; and after anastomosis, the PV and SMV should be retained to avoid distortion.
In the past 2 years, we have 11 patients involved in this study; in the future, we have suitable patients to continue it to increase the data and improve the study.
Conclusions
In summary, we believe that the use of the peritoneum and the round ligament of the liver to reconstruct the PV and SMV offers the following advantages: (I) in patients undergoing laparotomy, it is convenient to collect the materials without additional incisions; (II) the length of these vessels is sufficient to meet the needs for reconstruction; (III) these tissues are autologous and require no anticoagulant therapy or immunotherapy after surgery. Thus, the peritoneum and the round ligament of the liver are feasible clinical options available for reconstruction of the PV and SMV.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-712
Data Sharing Statement: Available at https://dx.doi.org/10.21037/gs-21-712
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-712). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Human Research Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (No. 2021 0939) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kriger AG, Karmazanovsky GG, Smirnov AV, et al. Diagnosis and treatment of pancreatic head cancer followed by mesenteric-portal vein invasion. Khirurgiia (Mosk) 2018;21-9. [Crossref] [PubMed]
- Zhang XM, Fan H, Kou JT, et al. Resection of portal and/or superior mesenteric vein and reconstruction by using allogeneic vein for pT3 pancreatic cancer. J Gastroenterol Hepatol 2016;31:1498-503. [Crossref] [PubMed]
- Hirono S, Kawai M, Tani M, et al. Indication for the use of an interposed graft during portal vein and/or superior mesenteric vein reconstruction in pancreatic resection based on perioperative outcomes. Langenbecks Arch Surg 2014;399:461-71. [Crossref] [PubMed]
- Batool S, Malik AA, Bari H, et al. Vascular Resection and Reconstruction in Pancreatic Tumours. J Coll Physicians Surg Pak 2018;28:485-7. [Crossref] [PubMed]
- Zhai S, Huo Z, Wang Y, et al. TRIANGLE operation for borderline resectable pancreatic cancer in total pancreatectomy. Transl Cancer Res 2019;8:2416-24. [Crossref]
- Schneider M, Strobel O, Hackert T, et al. Pancreatic resection for cancer-the Heidelberg technique. Langenbecks Arch Surg 2019;404:1017-22. [Crossref] [PubMed]
- Fang JZ, Lu CD, Wu SD, et al. Portal vein/superior mesenteric vein resection in pancreatic cancer treatment in the elderly. Medicine (Baltimore) 2017;96:e7335 [Crossref] [PubMed]
- Marino MV, Giovinazzo F, Podda M, et al. Robotic-assisted pancreaticoduodenectomy with vascular resection. Description of the surgical technique and analysis of early outcomes. Surg Oncol 2020;35:344-50. [Crossref] [PubMed]
- Liles JS, Katz MH. Pancreaticoduodenectomy with vascular resection for pancreatic head adenocarcinoma. Expert Rev Anticancer Ther 2014;14:919-29. [Crossref] [PubMed]
- Flis V, Potrc S, Kobilica N, et al. Pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head with venous resection. Radiol Oncol 2016;50:321-8. [Crossref] [PubMed]
- Brasoveanu V, Romanescu D, Barbu I, et al. Pancreatectomy With Arterial and Portal Vein Reconstruction for Locally Advanced Pancreatic Cancer - A Case Report and Literature Review. In Vivo 2020;34:2791-5. [Crossref] [PubMed]
- Barth U, May JP, Albrecht R, et al. Options and Management of Vascular Reconstruction in the Context of Abdominal Surgery and its Perioperative Care - Selection of Typical Clinical Situations and Cases. Zentralbl Chir 2019;144:460-70. [PubMed]
- Klose J, Hackert T, Büchler MW, et al. Vascular resection and reconstruction techniques in pancreatic surgery. Chirurg 2016;87:94-9. [Crossref] [PubMed]
- Ono Y, Tanaka M, Matsueda K, et al. Techniques for splenic vein reconstruction after pancreaticoduodenectomy with portal vein resection for pancreatic cancer. HPB (Oxford) 2019;21:1288-94. [Crossref] [PubMed]
- Liao K, Wang H, Chen Q, et al. Prosthetic graft for superior mesenteric-portal vein reconstruction in pancreaticoduodenectomy: a retrospective, multicenter study. J Gastrointest Surg 2014;18:1452-61. [Crossref] [PubMed]
- Meniconi RL, Santoro R, Guglielmo N, et al. Pancreaticoduodenectomy with venous reconstruction using cold-stored vein allografts: long-term results of a single center experience. J Hepatobiliary Pancreat Sci 2016;23:43-9. [Crossref] [PubMed]
- Oh CS, Won HS, Kwon CH, et al. Morphologic variations of the umbilical ring, umbilical ligaments and ligamentum teres hepatis. Yonsei Med J 2008;49:1004-7. [Crossref] [PubMed]




