
Diagnostic value of C-reactive protein and procalcitonin for postoperative pancreatic fistula following pancreatoduodenectomy: a systematic review and meta-analysis
Introduction
Pancreaticoduodenectomy (PD) is one of the standard surgical methods used to treat ampullary malignant tumors such as carcinoma of the pancreatic head, carcinoma of the lower common bile duct, and carcinoma of the duodenal papilla (1,2). However, postoperative complications still greatly affect patient recovery, presenting an urgent problem for hepatobiliary and pancreatic surgeons. Pancreatic fistula (PF) is a common and potentially severe complication after PD (3,4). Soft pancreas is an independent risk factor for PF after PD. The main pancreatic duct of soft pancreas is often thin, which makes it difficult to operate and the suture is not firm during PD, thus increasing the risk of PF after PD (2).
Relevant literature shows that the incidence of PF after PD is as high as 30% (5). Furthermore, the occurrence of PF after PD usually leads to other complications (6,7). Both grade B and grade C PF are defined as clinical postoperative pancreatic fistula (POPF) (8). Grade B PF is usually accompanied by abdominal hemorrhage and abdominal infection, while the more severe grade C PF is usually accompanied by organ failure or further complications leading to death. In addition, the occurrence of a POPF will extend the patient’s postoperative hospital stay, affect their postoperative healing, and increase the financial and mental burden placed upon them (9,10).
POPF is a serious postoperative complication, and reducing the incidence of POPF is one of the most effective means to reducing further complications after PD (11). Although there have been a large number of studies on the risk factors of POPF after PD, there is still much controversy, and it is difficult to determine all the clinical variables and related risk factors (12,13). Markers for POPF include procalcitonin (PCT), C-reactive protein (CRP), drain amylase, serum lipase, serum amylase, and white blood cells (WBCs) (14-20). The difficulty arises from the sheer number of factors related to POPF, making it impossible to resolve in a single study.
At present, other systematic reviews have studied the diagnostic value of drain amylase and WBC in predicting POPF after PD (21-24), but there are few systematic reviews on the diagnostic value of CRP and PCT in such cases. CRP is a sensitive marker for judging tissue damage and inflammation. PCT is a marker with high specificity to determine bacterial infection. The purpose of this study is to evaluate the diagnostic value of CRP and PCT in predicting POPF after PD by meta-analysis. We present the following article in accordance with the PRISMA-DTA reporting checklist (available at https://dx.doi.org/10.21037/gs-21-658).
Methods
Literature search strategy
Electronic databases, including PubMed, Excerpta Medica (EMBASE), the Web of Science (WOS), and the China National Knowledge Infrastructure (CNKI) were systematically searched from inception to July 2021 for the following keywords: (I) C-reactive protein (CRP); (II) procalcitonin (PCT); (III) postoperative pancreatic fistula (POPF); and (IV) pancreatoduodenectomy (PD). To broaden the search, numerous combinations of words and strings were applied with the Boolean operators “AND” and “OR”. There were no restrictions on the language of publication in document retrieval. To identify additional eligible studies, we reviewed reference lists from eligible trials and relevant reviews and guidelines. Disagreements were resolved through consensus between the two reviewers.
POPF definition
POPF is defined as any measurable excretion volume with an amylase content greater than three times the upper limit of normal serum, and classified as follows (25):
- Grade A: a temporary fistula with no clinical impact; patient can take orally, and clinical condition is good;
- Grade B: patient receives partial or total parenteral or enteral nutrition support, and usually requires continuous drainage for 3 weeks;
- Grade C: clinical management of fistula changes significantly or deviates from the normal clinical pathway, and radiation intervention or reoperation is required.
In our study, grade B and grade C were defined as clinical POPF.
Study selection
In selecting studies for inclusion in this meta-analysis, we applied the following criteria:
- Study is about patients with PD;
- Study focuses on the value of CRP and/or PCT in the diagnosis of POPF after PD;
- Study directly or indirectly provides the following data: true positive (TP), false positive (FP), false negative (FN) and true negative (TN);
- Study is available in full text.
The exclusion criteria agreed upon were as follows:
- Study does not meet the inclusion criteria;
- Relevant results are not reported or cannot be used;
- Only review or abstract is available, or study is a duplicate publication.
Data extraction and quality assessment
The data extracted from the selected studies included: year of publication, country of origin, sample size, patient age, POPF prevalence, reference standard, and tested variables. The validity of the eligible studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 tool (QUADAS-2) in the RevMan software suite (version 5.4). Data extraction and quality assessment were performed independently by two reviewers, and disagreements were resolved by consensus.
Statistical analysis
The meta-analysis in our study was performed using RevMan 5.4 (The Cochrane Collaboration, Oxford, UK) and STATA 14.0 (STATA Corp., College Station, TX, USA). We evaluated the degree of statistical heterogeneity and inconsistency by using the Chi 2 and I2 statistics. The random-effect model was applied if heterogeneity was observed, while the fixed-effect model was applied in the absence of between-study heterogeneity. In the meta-analysis of diagnostic test accuracy, the threshold effect is one of the important reasons for the heterogeneity. Therefore, if there is a threshold effect, the best way to merge data is to fit the summary receiver operating characteristic (SROC) curve and calculate the area under the SROC curve (AUC) when performing a Meta-analysis to merge effect values. In this study, we all use SROC curve and AUC to judge the diagnostic value. Sensitivity analysis was conducted by eliminating individual studies one by one, and Deeks’ funnel plot was used to identify publication bias when the number of articles included exceeded 10. P>0.05 was considered indicative of no significant publication bias.
Results
Search process
A total of 1,322 potentially relevant articles from electronic databases were retrieved after the literature search. By preliminary screening of the titles and abstracts, we excluded 1,160 documents, which did not meet the inclusion criteria. After full-text screening, a further 142 articles were excluded. Thus, 20 studies met the criteria for inclusion in the present meta-analysis (26-45). The process of literature retrieval is shown in Figure 1.
Characteristics of included studies
The baseline characteristics of the studies included in the meta-analysis are shown in Table 1. This study includes ten prospective cohort studies and ten retrospective cohort studies consisting of 4,076 patients. All articles were published from 2013 to 2021. POPF prevalence ranged from 7.5% to 26%. All studies adopted the standard of the International Study Group of Pancreatic Surgery (ISGPS) as the reference standard for POPF.
Table 1
| Study | Country | Study design | No. of patients | Gender (M/F) | Age, years | POPF prevalence | Study interval | Reference standard | Tested variables |
|---|---|---|---|---|---|---|---|---|---|
| Fujiwara 2013 | Japan | Retrospective | 297 | 181/116 | 63.4 [13–86] | 64 (22%) | January 2001 to December 2011 | ISGPF | CRP |
| Hiyoshi 2013 | Japan | Prospective | 176 | 108/68 | – | 30 (17%) | March 2002 to December 2010 | ISGPF | CRP |
| Kosaka 2013 | Japan | Retrospective | 100 | 64/36 | – | 32 (32%) | January 2009 to October 2012 | ISGPF | CRP |
| Ansorge 2014 | Sweden | Prospective | 315 | 141/174 | 67 [22–87] | 48 (15.2%) | January 2008 to June 2012 | ISGPF | CRP |
| Uemura 2014 | Japan | Prospective | 200 | 115/85 | 68 [19–88] | 15 (7.5%) | April 2004 to June 2011 | ISGPF | CRP |
| Solaini 2015 | UK | Prospective | 378 | 183/195 | 65 [52–72] | 31 (8.2%) | January 2005 to December 2012 | ISGPF | CRP |
| Giardino 2016 | Italy | Prospective | 84 | 47/37 | 64 [56–72] | 18 (21.4%) | January 2015 to November 2015 | ISGPF | CRP/PCT |
| Palani Velu 2016 | UK | Prospective | 230 | 151/79 | – | 54 (23.5%) | January 2008 to January 2014 | ISGPF | CRP |
| Bai 2017 | China | Prospective | 87 | 53/34 | 62±10 | 18 (20.7%) | March 2016 to December 2016 | ISGPF | PCT |
| Partelli 2017 | Italy | Prospective | 463 | 261/202 | 68 [17–85] | 64 (14%) | 2013 to 2015 | ISGPF | CRP |
| Guilbaud 2018 | France | Prospective | 110 | 61/49 | 65 [24–85] | 24 (22%) | January 2013 to November 2016 | ISGPF | CRP |
| Malya 2018 | Turkey | Retrospective | 117 | 71/46 | 60.7±13.3 | 9 (8.7%) | 2012 to 2015 | ISGPF | CRP |
| Li 2019 | China | Retrospective | 62 | 41/21 | – | 12 (19.4%) | April 2016 to April 2017 | ISGPF | PCT |
| Mario 2019 | Spain | Prospective | 50 | 29/21 | – | 13 (26%) | January 2015 to March 2018 | ISGPF | CRP |
| Uchida 2019 | Japan | Retrospective | 211 | 126/85 | 68 [22–85] | 38 (18%) | 2012 to 2018 | ISGPF | CRP |
| Dongen 2020 | Netherlands | Retrospective | 202 | 110/92 | 68 [59–74[ | 35 (17.3%) | January 2012 to December 2017 | ISGPF | CRP |
| Mintziras 2020 | Germany | Retrospective | 188 | 98/90 | 67.5 [56.3–75] | 30 (16%) | January 2009 to December 2018 | ISGPF | CRP/PCT |
| Zhou 2020 | China | Retrospective | 67 | 43/24 | – | 14 (20.9%) | January 2017 to December 2018 | ISGPF | PCT |
| Farooqui 2021 | Denmark | Retrospective | 552 | 281/271 | 69 [16–90] | 48 (8.7%) | January 2015 to December 2019 | ISGPF | CRP |
| Ma 2021 | China | Retrospective | 186 | 102/84 | 61 [52–67] | 18 (9.7%) | January 2019 to November 2019 | ISGPF | CRP/PCT |
POPF, postoperative pancreatic fistula; ISGPS, International Study Group of Pancreatic Surgery; CRP, C-reactive protein; PCT, procalcitonin.
Results of quality assessment
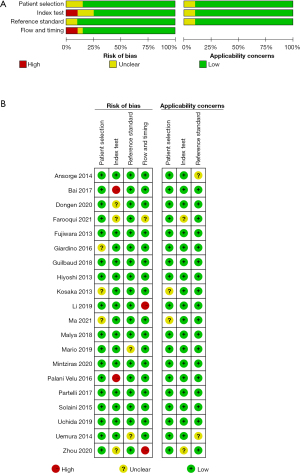
As shown in Figure 2, the QUADAS-2 tool was used to assess the quality of the selected studies, among which two showed a high risk of index test bias, and two others showed a high risk of flow and timing bias. A summary of the risk of bias assessment for each study is shown in Figure 2B.
Results of diagnostic accuracy
Given the significant changes in PCT and CRP after PD and the likelihood of different diagnostic values on different postoperative days (PODs), we carried out a subgroup analysis according to POD.
Diagnostic accuracy of CRP
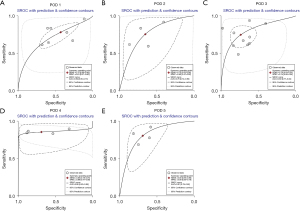
Table 2 shows the results of the meta-analysis of the diagnostic accuracy of CRP in predicting POPF after PD, presented separately for POD 1–5. The highest sensitivity was found on POD 4 (0.85; 95% CI: 0.78–0.91); the highest specificity occurred on POD 3 (0.74; 95% CI: 0.60–0.84); and the highest diagnostic odds ratio (DOR) was found on POD 4 (13; 95% CI: 4–41). Regarding sensitivity and specificity, the highest AUC was also found on POD 4 (0.86; 95% CI: 0.83–0.89). Figure 3 shows the AUC on POD 1–5.
Table 2
| Study | POPF prevalence | Cut-off values (mg/L) | Sensitivity | Specificity | AUC | Pooled sensitivity (95% CI) | Pooled specificity (95% CI) | Pooled DOR (95% CI) | Pooled AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| POD 1 | |||||||||
| Guilbaud 2018 | 24 (22%) | 100 | 0.84 | 0.61 | 0.72 | 0.78 (0.66–0.86) | 0.43 (0.27–0.60) | 3 [2–4] | 0.69 (0.65–0.73) |
| Malya 2018 | 9 (8.7%) | 55 | 0.7 | 0.27 | 0.407 | ||||
| Ma 2021 | 18 (9.7%) | 65 | 0.625 | 0.684 | 0.625 | ||||
| Giardino 2016 | 18 (21.4%) | 92 | 0.87 | 0.57 | 0.72 | ||||
| Palani Velu 2016 | 54 (23.5%) | 98 | 0.796 | 0.347 | 0.573 | ||||
| Fujiwara 2013 | 64 (22%) | 94 | 0.643 | 0.596 | 0.644 | ||||
| Farooqui 2021 | 48 (8.7%) | 64 | 0.959 | 0.117 | 0.582 | ||||
| POD 2 | |||||||||
| Mario 2019 | 13 (26%) | 250 | 0.69 | 0.87 | 0.79 | 0.75 (0.55–0.88) | 0.65 (0.47–0.80) | 6 [3–13] | 0.76 (0.72–0.80) |
| Ma 2021 | 18 (9.7%) | 217 | 0.875 | 0.715 | 0.794 | ||||
| Palani Velu 2016 | 54 (23.5%) | 230 | 0.611 | 0.619 | 0.682 | ||||
| Farooqui 2021 | 48 (8.7%) | 114 | 0.931 | 0.408 | 0.668 | ||||
| POD 3 | |||||||||
| Dongen 2020 | 35 (17.3%) | 200 | 0.72 | 0.62 | 0.79 | 0.74 (0.63–0.83) | 0.74 (0.60–0.84) | 8 [4–17] | 0.80 (0.77–0.84) |
| Ansorge 2014 | 48 (15.2%) | 200 | 0.78 | 0.83 | 0.854 | ||||
| Partelli 2017 | 64 (14%) | 185 | 0.94 | 0.62 | 0.796 | ||||
| Solaini 2015 | 31 (8.2%) | 272 | 0.50 | 0.77 | 0.644 | ||||
| Malya 2018 | 9 (8.7%) | 225 | 0.70 | 0.71 | 0.668 | ||||
| Hiyoshi 2013 | 30 (17%) | 200 | 0.846 | 0.982 | 0.843 | ||||
| Mintziras 2020 | 30 (16%) | 203 | 0.63 | 0.84 | 0.81 | ||||
| Ma 2021 | 18 (9.7%) | 201 | 0.647 | 0.643 | 0.629 | ||||
| Palani Velu 2016 | 54 (23.5%) | 204 | 0.63 | 0.624 | 0.692 | ||||
| Farooqui 2021 | 48 (8.7%) | 122 | 0.906 | 0.404 | 0.762 | ||||
| POD 4 | |||||||||
| Kosaka 2013 | 32 (32%) | 93 | 0.86 | 0.89 | 0.90 | 0.85 (0.78–0.91) | 0.69 (0.41–0.88) | 13 [4–41] | 0.86 (0.83–0.89) |
| Uemura 2014 | 15 (7.5%) | 156 | 0.867 | 0.87 | 0.866 | ||||
| Palani Velu 2016 | 54 (23.5%) | 134 | 0.834 | 0.538 | 0.708 | ||||
| Farooqui 2021 | 48 (8.7%) | 62 | 0.912 | 0.319 | 0.781 | ||||
| POD 5 | |||||||||
| Dongen 2020 | 35 (17.3%) | 150 | 0.71 | 0.75 | 0.78 | 0.80 (0.68–0.89) | 0.69 (0.59–0.78) | 9 [5–16] | 0.81 (0.78–0.85) |
| Malya 2018 | 9 (8.7%) | 190 | 0.9 | 0.822 | 0.851 | ||||
| Uchida 2019 | 38 (18%) | 50 | 0.941 | 0.585 | 0.802 | ||||
| Ma 2021 | 18 (9.7%) | 95 | 0.813 | 0.603 | 0.702 |
POPF, postoperative pancreatic fistula; AUC, area under the curve; DOR, diagnostic odds ratios.
Diagnostic accuracy of PCT
Table 3 shows the results of the meta-analysis of the diagnostic accuracy of PCT in predicting POPF after PD, presented separately for POD 1, 3 and 5, as no study chose to display the diagnostic accuracy of PCT on POD 4, and only 1 study provided data for POD 2, making it impossible to conduct a pooled analysis of POD 2 and POD 4. The results show that the highest sensitivity was found on POD 1 (0.84; 95% CI: 0.72–0.91) and POD 5 (0.84; 95% CI: 0.71–0.92); the highest specificity occurred on POD 3 (0.77; 95% CI: 0.61–0.87); and the highest DOR was found on POD 5 (15; 95% CI: 5–44). Regarding sensitivity and specificity, the highest AUC was also found on POD 5 (0.87; 95% CI: 0.84–0.90). Figure 4 shows the AUC on POD 1, 3 and 5.
Table 3
| Study | POPF prevalence | Cut-off values (μg/L) | Sensitivity | Specificity | AUC | Pooled sensitivity (95% CI) | Pooled specificity (95% CI) | Pooled DOR (95% CI) | Pooled AUC |
|---|---|---|---|---|---|---|---|---|---|
| POD 1 | |||||||||
| Zhou 2020 | 14 (20.8%) | 0.67 | 0.737 | 0.761 | 0.77 | 0.84 (0.72–0.91) | 0.70 (0.54–0.82) | 12 [5–28] | 0.86 (0.82–0.88) |
| Ma 2021 | 18 (9.7%) | 0.65 | 0.813 | 0.759 | 0.788 | ||||
| Giardino 2016 | 18 (21.4%) | 0.4 | 0.93 | 0.43 | 0.70 | ||||
| Li 2019 | 12 (19.4%) | 0.38 | 1 | 0.80 | 0.92 | ||||
| POD 2 | |||||||||
| Ma 2021 | 18 (9.7%) | 3.3 | 0.813 | 0.937 | 0.931 | ||||
| POD 3 | |||||||||
| Zhou 2020 | 14 (20.8%) | 0.56 | 0.895 | 0.642 | 0.83 | 0.74 (0.59–0.85) | 0.77 (0.61–0.87) | 9 [4–24] | 0.81 (0.78–0.84) |
| Mintziras 2020 | 30 (16%) | 0.85 | 0.52 | 0.83 | 0.77 | ||||
| Ma 2021 | 18 (9.7%) | 2.1 | 0.882 | 0.929 | 0.951 | ||||
| Bai 2017 | 18 (20.7%) | 0.259 | 0.778 | 0.537 | 0.689 | ||||
| Li 2019 | 12 (19.4%) | 0.44 | 0.833 | 0.74 | 0.89 | ||||
| POD 4 | |||||||||
| None | |||||||||
| POD 5 | |||||||||
| Zhou 2020 | 14 (20.8%) | 0.46 | 0.684 | 0.761 | 0.72 | 0.84 (0.71–0.92) | 0.74 (0.57–0.86) | 15 [5–44] | 0.87 (0.84–0.90) |
| Ma 2021 | 18 (9.7%) | 0.91 | 0.938 | 0.879 | 0.930 | ||||
| Bai 2017 | 18 (20.7%) | 0.126 | 0.889 | 0.475 | 0.723 | ||||
| Li 2019 | 12 (19.4%) | 0.98 | 0.917 | 0.76 | 0.84 |
POPF, postoperative pancreatic fistula; AUC, area under the curve; DOR, diagnostic odds ratios; POD, postoperative day.

Sensitivity analysis
We conducted a sensitivity analysis by excluding the selected studies one by one and observing whether the results obtained changed significantly. No noticeable changes were observed, indicating that these studies are relatively stable.
Publication bias
We evaluated the publication bias of the selected studies using Deeks’ funnel plot asymmetry test for the diagnostic value of CRP on POD 3. The P value was 0.70, which indicates that no significant publication bias exists in this meta-analysis (Figure 5).
Discussion
POPF is a severe complication of PD, with a high rate of morbidity. The clinically relevant POPF is often accompanied by abdominal cavity infection. Abdominal cavity infection is not only related to POPF, but may also be an important inducing and aggravating factor for the occurrence and development of POPF, but the exact correlation between abdominal cavity infection and POPF is not clear (7).
Current literature shows that many factors determine the risk of developing a PF after PD, including gender, body mass index, diabetes, pancreatic texture, pancreatic duct diameter, intraoperative blood loss, pathological type, anastomosis, neoadjuvant therapy, somatostatin analogues and drainage tube placement (46,47). Based on the above risk factors, several risk prediction scoring systems for POPF have been established, such as the National Cancer Center Hospital (NCCH) POPF prediction scoring system, the Fistula Risk Score (FRS), and the measurement of amylase in postoperative drainage fluid (48,49).
To find a simple and accurate method for assessing POPF, early screening and effective preventive measures for high-risk patients have been the focus of pancreatic surgeons. The detection of blood indexes with high sensitivity and specificity is one of the most reliable predictors of POPF (50). Therefore, clarifying the correlation between early postoperative biochemical sensitive indexes and POPF is of significant clinical value.
Several markers, such as drain amylase, serum lipase, serum amylase and WBC, have been proposed as predictors for POPF (11,17,20,51). Yang et al. (52) found that a value of drain fluid amylase on POD 1 over 1,300 U/L indicated a risk factor for PF, with a pooled sensitivity and specificity of 81% and 87%, respectively. Liu et al. (53) concluded that the drain/plasma pancreatic amylase value on POD 1 was a useful predictive test for overall POPF and clinical POPF with high sensitivity (92%) and specificity (77%) scores. The present meta-analysis aimed to assess the accuracy of CRP and PCT in the prediction of POPF. To date, there have been few studies to assess the pooled performance of CRP and PCT for POPF after PD.
It was found that both CRP and PCT are effective in helping to diagnose POPF and have a high AUC. They could therefore be used as a routine means for diagnosing POPF. Regarding the AUC value: an AUC of 0.5–0.7 suggests low diagnostic accuracy; AUC of 0.7–0.9 suggests medium diagnostic accuracy; and AUC >0.9 suggests high diagnostic accuracy. Our study showed that the AUC of CRP on POD 3–5 was greater than 0.80, especially the AUC of CRP on POD 4, which had a value of 0.86, with a sensitivity and specificity of 0.85 and 0.69, respectively, illustrating the positive diagnostic value of CRP for POPF. The AUC of PCT on POD 5 also showed high diagnostic accuracy for POPF with a value of 0.87, and a sensitivity and specificity of 0.84 and 0.74, respectively.
Several limitations should be noted. The ISGPF published two slightly different versions of POPF in 2005 and 2016 (54,55). Most of the studies included in this meta-analysis adopted the 2005 version, although some adopted the 2016 version, which may impact the results. Secondly, there were differences in the cutoff values of CRP and PCT in each study, which will have a certain impact on the final sensitivity and specificity and consequently affect the results of the AUC. Thirdly, the number of studies on the diagnosis of POPF by PCT was small, and more studies are needed to confirm its accuracy.
In conclusion, CRP and PCT have a high diagnostic value in predicting POPF, especially the CRP levels on POD 4 and PCT levels on POD 5. Given the study’s limitations, more randomized controlled trials should be implemented to provide further unbiased evidence.
Acknowledgments
Funding: This work was supported by research grants from the science and technology plan project for research and development in Chengde (No. 201804A039).
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-658
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-658). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fabre JM, Burgel JS, Navarro F, et al. Delayed gastric emptying after pancreaticoduodenectomy and pancreaticogastrostomy. Eur J Surg 1999;165:560-5. [Crossref] [PubMed]
- Pu N, Mu S, Fang Y, et al. Survival outcomes of pancreaticoduodenectomy versus extended pancreaticoduodenectomy procedure for pancreatic head carcinoma: a propensity score matching study. Transl Cancer Res 2020;9:1476-86. [Crossref]
- Jagannath P, Dhir V, Shrikhande S, et al. Effect of preoperative biliary stenting on immediate outcome after pancreaticoduodenectomy. Br J Surg 2005;92:356-61. [Crossref] [PubMed]
- Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol 2011;18:2126-35. [Crossref] [PubMed]
- Akiyama Y, Fujimoto K, Komatsubara T, et al. Surgical Apgar Score for Prediction of Morbidity after Pancreatectomy. The Japanese Journal of Gastroenterological Surgery 2016;49:842-9. [Crossref]
- Yang C, Guan S, Wang J, et al. Effects on inflammatory factors and immune indexes between enteral nutrition and parenteral nutrition support in hepatobiliary surgery patients. Journal of Nurses Training 2017;24:16-19.
- Boteon APCS, Boteon YL, Hodson J, et al. Multivariable analysis of predictors of unplanned hospital readmission after pancreaticoduodenectomy: development of a validated risk score. HPB (Oxford) 2019;21:26-33. [Crossref] [PubMed]
- Sakamoto T, Yagyu Y, Uchinaka EI, et al. Predictive Significance of C-reactive Protein-to-albumin Ratio for Postoperative Pancreatic Fistula After Pancreaticoduodenectomy. Anticancer Res 2019;39:6283-90. [Crossref] [PubMed]
- Iwasaki T, Nara S, Kishi Y, et al. Proposal of a Clinically Useful Criterion for Early Drain Removal After Pancreaticoduodenectomy. J Gastrointest Surg 2021;25:737-46. [Crossref] [PubMed]
- Wellner UF, Sick O, Olschewski M, et al. Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg 2012;16:1686-95. [Crossref] [PubMed]
- Iida H, Maehira H, Mori H, et al. Serum procalcitonin as a predictor of infectious complications after pancreaticoduodenectomy: review of the literature and our experience. Surg Today 2020;50:87-96. [Crossref] [PubMed]
- Shiba H, Misawa T, Fujiwara Y, et al. Glasgow prognostic score predicts therapeutic outcome after pancreaticoduodenectomy for carcinoma of the ampulla of vater. Anticancer Res 2013;33:2715-21. [PubMed]
- Räty S, Sand J, Lantto E, et al. Postoperative acute pancreatitis as a major determinant of postoperative delayed gastric emptying after pancreaticoduodenectomy. J Gastrointest Surg 2006;10:1131-9. [Crossref] [PubMed]
- Matsumoto M, Nakabayashi Y, Fujiwara Y, et al. Duration of Preoperative Biliary Drainage as a Prognostic Factor After Pancreaticoduodenectomy for Pancreatic Head Cancer. Anticancer Res 2017;37:3215-9. [PubMed]
- Sanjay P, de Figueiredo RS, Leaver H, et al. Preoperative serum C-reactive protein levels and post-operative lymph node ratio are important predictors of survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. JOP 2012;13:199-204. [PubMed]
- Sushma N, Gupta P, Kumar H, et al. Role of ultrasound shear wave elastography in preoperative prediction of pancreatic fistula after pancreaticoduodenectomy. Pancreatology 2020;20:1764-9. [Crossref] [PubMed]
- Segersv R, Blomberg J, Chiaro MD, et al. The diagnostic value of abdominal drainage in the individual risk assessment for pancreatic fistula following pancreaticoduodenectomy. Pancreatology 2013;13:S8-S9. [Crossref]
- Zhou Y, Dai T, Hua Z, et al. The Effect of Application of Accelerated Rehabilitation Surgery during Perioperative Period of Pancreaticoduodenectomy in Elderly Patients. Journal of Guizhou Medical University 2017;10:1215-8.
- Velu L, Chandrabalan V, Carter R, et al. The Glasgow Whipple risk score to predict pancreas-specific complications after pancreaticoduodenectomy. J Clin Oncol 2015;33:394. [Crossref]
- Qu G, Wang D, Xu W, et al. The Systemic Inflammation-Based Prognostic Score Predicts Postoperative Complications in Patients Undergoing Pancreaticoduodenectomy. Int J Gen Med 2021;14:787-95. [Crossref] [PubMed]
- Smits FJ, Molenaar IQ, Besselink MG, et al. Early recognition of clinically relevant postoperative pancreatic fistula: a systematic review. HPB (Oxford) 2020;22:1-11. [Crossref] [PubMed]
- Giglio MC, Spalding DR, Giakoustidis A, et al. Meta-analysis of drain amylase content on postoperative day 1 as a predictor of pancreatic fistula following pancreatic resection. Br J Surg 2016;103:328-36. [Crossref] [PubMed]
- Kamarajah SK, Bundred JR, Lin A, et al. Systematic review and meta-analysis of factors associated with post-operative pancreatic fistula following pancreatoduodenectomy. ANZ J Surg 2021;91:810-21. [Crossref] [PubMed]
- Lu X, Wang X, Fang Y, et al. Systematic Review and Meta-Analysis of Pancreatic Amylase Value on Postoperative Day 1 After Pancreatic Resection to Predict Postoperative Pancreatic Fistula. Medicine (Baltimore) 2016;95:e2569. [Crossref] [PubMed]
- Angiolini MR, Gavazzi F, Ridolfi C, et al. Role of C-Reactive Protein Assessment as Early Predictor of Surgical Site Infections Development after Pancreaticoduodenectomy. Dig Surg 2016;33:267-75. [Crossref] [PubMed]
- Fujiwara Y, Misawa T, Shiba H, et al. A novel postoperative inflammatory score predicts postoperative pancreatic fistula after pancreatic resection. Anticancer Res 2013;33:5005-10. [PubMed]
- Bai S, Sheng L, Zheng K, et al. A prospective study on the predictive value of procalcitonin for postoperative complications after pancreaticoduodenectomy. Chin J Pancreatol 2017;17:104-8.
- Uchida Y, Masui T, Nakano K, et al. Combination of postoperative C-reactive protein value and computed tomography imaging can predict severe pancreatic fistula after pancreatoduodenectomy. HPB (Oxford) 2020;22:282-8. [Crossref] [PubMed]
- Mintziras I, Maurer E, Kanngiesser V, et al. C-reactive protein and drain amylase accurately predict clinically relevant pancreatic fistula after partial pancreaticoduodenectomy. Int J Surg 2020;76:53-8. [Crossref] [PubMed]
- Giardino A, Spolverato G, Regi P, et al. C-Reactive Protein and Procalcitonin as Predictors of Postoperative Inflammatory Complications After Pancreatic Surgery. J Gastrointest Surg 2016;20:1482-92. [Crossref] [PubMed]
- van Dongen JC, Smits FJ, van Santvoort HC, et al. C-reactive protein is superior to white blood cell count for early detection of complications after pancreatoduodenectomy: a retrospective multicenter cohort study. HPB (Oxford) 2020;22:1504-12. [Crossref] [PubMed]
- Guilbaud T, Birnbaum DJ, Lemoine C, et al. C-Reactive Protein on Postoperative Day 1 Is a Reliable Predictor of Pancreas-Specific Complications After Pancreaticoduodenectomy. J Gastrointest Surg 2018;22:818-30. [Crossref] [PubMed]
- Ansorge C, Nordin JZ, Lundell L, et al. Diagnostic value of abdominal drainage in individual risk assessment of pancreatic fistula following pancreaticoduodenectomy. Br J Surg 2014;101:100-8. [Crossref] [PubMed]
- Partelli S, Pecorelli N, Muffatti F, et al. Early Postoperative Prediction of Clinically Relevant Pancreatic Fistula after Pancreaticoduodenectomy: usefulness of C-reactive Protein. HPB (Oxford) 2017;19:580-6. [Crossref] [PubMed]
- Rodriguez-Lopez M, Tejero-Pintor FJ, Bailon-Cuadrado M, et al. Impaired immune reaction and increased lactate and C-reactive protein for early prediction of severe morbidity and pancreatic fistula after pancreatoduodenectomy. Hepatobiliary Pancreat Dis Int 2020;19:58-67. [Crossref] [PubMed]
- Uemura K, Murakami Y, Sudo T, et al. Indicators for proper management of surgical drains following pancreaticoduodenectomy. J Surg Oncol 2014;109:702-7. [Crossref] [PubMed]
- Solaini L, Atmaja BT, Watt J, et al. Limited utility of inflammatory markers in the early detection of postoperative inflammatory complications after pancreatic resection: Cohort study and meta-analyses. Int J Surg 2015;17:41-7. [Crossref] [PubMed]
- Kosaka H, Kuroda N, Suzumura K, et al. Multivariate logistic regression analysis for prediction of clinically relevant pancreatic fistula in the early phase after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2014;21:128-33. [Crossref] [PubMed]
- Ma J, Jiang P, Ji B, et al. Post-operative procalcitonin and C-reactive protein predict pancreatic fistula after laparoscopic pancreatoduodenectomy. BMC Surg 2021;21:171. [Crossref] [PubMed]
- Li G, Guo D, Shang Y, et al. Predictive value of procalcitonin for postoperative early pancreatic fistula. Chin J Gen Surg 2019;34:108.
- Palani Velu LK, McKay CJ, Carter CR, et al. Serum amylase and C-reactive protein in risk stratification of pancreas-specific complications after pancreaticoduodenectomy. Br J Surg 2016;103:553-63. [Crossref] [PubMed]
- Farooqui W, Riemenschneider KA, Penninga L, et al. The diagnostic value of C-reactive protein for predicting pancreatic fistula following pancreatoduodenectomy. Scand J Gastroenterol 2021;56:329-35. [Crossref] [PubMed]
- Zhou Q, Xia Y, Lei Z. The predictive value of procalcitonin for postoperative early pancreatic fistula. BMC Surg 2020;20:90. [Crossref] [PubMed]
- Malya FU, Hasbahceci M, Tasci Y, et al. The Role of C-Reactive Protein in the Early Prediction of Serious Pancreatic Fistula Development after Pancreaticoduodenectomy. Gastroenterol Res Pract 2018;2018:9157806. [Crossref] [PubMed]
- Hiyoshi M, Chijiiwa K, Fujii Y, et al. Usefulness of drain amylase, serum C-reactive protein levels and body temperature to predict postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg 2013;37:2436-42. [Crossref] [PubMed]
- Yoo D, Park SY, Hwang DW, et al. Lack of Association between Postoperative Pancreatitis and Other Postoperative Complications Following Pancreaticoduodenectomy. J Clin Med 2021;10:1179. [Crossref] [PubMed]
- Lu X, Chen Y. Pathologic Assessment of Pancreatic Fibrosis in Predicting Pancreatic Fistula and Management of Prophylactic Drain Removal After Pancreaticoduodenectomy. World J Surg 2016;40:1520-1. [Crossref] [PubMed]
- Fukui T, Noda H, Watanabe F, et al. Drain output volume after pancreaticoduodenectomy is a useful warning sign for postoperative complications. BMC Surg 2021;21:279. [Crossref] [PubMed]
- Hata T, Mizuma M, Motoi F, et al. Serum procalcitonin as an early diagnostic marker of severe postoperative complications after elective pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2020;27:767-75. [Crossref] [PubMed]
- Ming S, Xue X, Qian J. Effects of Early Enteral Nutrition on Acute Inflammatory Response after Pancreaticoduodenectomy. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong 2006;2:256-8.
- Sakamoto K, Ogawa K, Inoue H, et al. Significant association between the preoperative erythrocyte mean corpuscular volume and infectious complications after pancreaticoduodenectomy. Surg Today 2021;51:258-67. [Crossref] [PubMed]
- Yang J, Huang Q, Wang C. Postoperative drain amylase predicts pancreatic fistula in pancreatic surgery: A systematic review and meta-analysis. Int J Surg 2015;22:38-45. [Crossref] [PubMed]
- Liu Y, Li Y, Wang L, et al. Predictive value of drain pancreatic amylase concentration for postoperative pancreatic fistula on postoperative day 1 after pancreatic resection: An updated meta-analysis. Medicine (Baltimore) 2018;97:e12487. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Chen BP, Bennett S, Bertens KA, et al. Use and acceptance of the International Study Group for Pancreatic Fistula (ISGPF) definition and criteria in the surgical literature. HPB (Oxford) 2018;20:69-75. [Crossref] [PubMed]
(English Language Editor: L. Roberts)




