
Tall cell carcinoma with reversed polarity: case report with gene sequencing and literature review
Introduction
Tall cell carcinoma with reversed polarity (TCCRP) is a rare subtype of breast cancer. It was first reported by Eusebi et al. in 2003 under the name of “breast tumor resembling the tall cell variant of papillary thyroid carcinoma (BTRPTC)” (1). Since subsequent studies found that it lacked the unique histologic and genetic properties of PTC, Masood et al. proposed that the terminology should be changed to “tall cell variant of papillary breast carcinoma (TCVPBC)” (2). In 2016, Chiang et al. pointed out that the tumor showed solid and papillary structures lined by columnar epithelial cells with “reverse polarity” significantly, and suggested it be renamed “solid papillary carcinoma with reverse polarization (SPCRP)” (3). In 2019, the World Health Organization (WHO) officially classified the tumor as “rare and salivary gland tumors”. TCCRP usually presents with well-circumscribed nodule, and is composed of solid and papillary architectures lined by tall columnar cells with nuclear grooves and nuclear pseudo-inclusions morphologically. The most characteristic feature of TCCRP, as it is called, is the apical location of the nuclei, displaying “reverse polarization”. Furthermore, the tumor often shows a triple-negative profile, with IDH2 and PIK3CA simultaneous gene mutations. Since TCCRP is generally believed as a low-invasive tumor with indolent behavior, the patients mainly undergo breast conserving surgery at present. However, there are still a few cases reported with local recurrence and distal metastasis. And no clear description was found about TCCRP in National Comprehensive Cancer Network (NCCN) clinical practice guidelines in breast cancer. Up to now, only 17 literatures and 75 cases of TCCRP have been reported, and further research should be conducted to determine the diagnosis and treatment criteria for this tumor (1-17).
Here, we report 2 cases of TCCRP, investigate their clinicopathological features, and analyze in terms of genetic mutations by using whole exome sequencing (WES) to confirm the specific mutations previously described as well as search for other meaningful genetic alterations. In addition, we reviewed all previous case reports of TCCRP in comparison with our cases. Typical clinicopathological features consistent with TCCRP and hotspot mutations (IDH2 and PIK3CA) were found in our cases.
We present the following cases in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-591).
Case presentation
Case 1
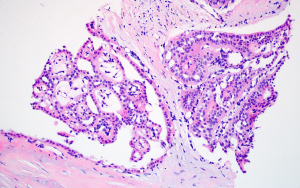
The patient was a 72-year-old woman with a palpable mass located in the upper outside quadrant of the right breast. The mammary ultrasonography revealed a well-circumscribed hypoechoic area of 2.3×1.7×1.7 cm3 in size at 9 o’clock, with an irregular shape, blood flow signals at the periphery, and a score of 5 under the Breast Imaging Reporting and Database System (BI-RADS). No significant abnormality was found in thyroid ultrasonography. The core biopsy specimen showed that most of the neoplastic cells were arranged in a papillary architecture, with the mesenchyme of tumor tissues accompanied by hyaline degeneration. The tumor cells were filled with eosinophilic cytoplasm, characterized by oval nuclei, clear chromatin, and occasional nuclear pseudo-inclusions (Figure 1). These results indicated a high possible diagnosis of TCCRP. The right breast mass was resected and sent for intraoperative freezing pathology later. Grossly, the cut surface showed a light red area of 2.2×2.2×1.8 cm3, with the macroscopic appearance of breast mass showed in Figure 2. A well-circumscribed and multinodular tissue was shown at low power. Under high magnification, it grew in a multi-nodular shape (Figure 3A). Because it was challenging to determine the existence of myoepithelial cells, we diagnosed it as papillary tumor, leaving the specific nature to be confirmed by postoperative paraffin pathology. Postoperative paraffin pathology confirmed the diagnosis of TCCRP and showed most of the tumor cells grew in nodular and infiltrating pattern with papillary architecture centered by fibrovascular cores. Some cystic spaces were filled with eosinophilic colloid-like material, accompanied by cystic dilatation. The neoplastic cells were columnar in shape, with eosinophilic cytoplasm, and showed grooves and eosinophilic pseudo-inclusions occasionally. Some nuclei are distributed near the lumen and the lumen edge of the cyst (Figure 3B-3D).

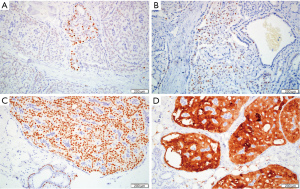
The immunohistochemistry (IHC) showed negative expression of SMMHC and P63, indicating the absence of myoepithelial cells around and within the tumor nests. Expression of ER was focal positive, with a rate of <1%. PR was negative and HER-2 expressed weak complete membrane staining observed in <10% of tumor cells. The Ki-67 proliferative index was around 5%. The cytoplasm of tumor cells showed diffuse reactivity for CK5 and calretinin and the nucleus of tumor cells diffusely expressed SOX-10, the cytoplasm of some tumor cells and the secretion in the dilated tube lumen were positive for GCDFP-15. The nucleus of a few tumor cells was positive for TTF-1. GATA-3 was focal positive and TG was negative. Laminin showed negative staining around the tumor nest. EMA showed positive staining in a few cytoplasm of tumor cells and the lumen edge (Figure 4A-4D). Subsequently, the patient underwent right mastectomy plus sentinel lymph node biopsy, and postoperative paraffin pathology showed no metastasis in axillary lymph nodes. Molecular genetic analysis was conducted using WES, and the results showed concurrent IDH2 p.R172S and PIK3CA p.H1047R hotspot mutations (Table S1). Chromosomal aberrations were not analyzed. The patient was alive and well for 6 months at the last follow-up.
Case 2
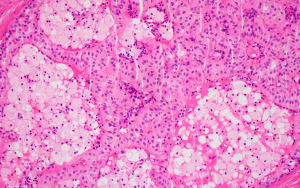
Our team has previously reported a 70-year-old woman with a palpable mass, measuring 2.0 cm in diameter, located in the upper outside quadrant of the left breast (18). The mammography revealed a nodule-like density increase area and no significant abnormality was found in thyroid ultrasonography. Wide local excision was conducted and sent for postoperative paraffin pathology. A gray-white, gray-yellow nodular mass of 1.6 cm in diameter with unclear boundary was found on the cut surface. Histologically, most of the tumor cells nuclei presented away from the stromal surface, giving the expression of reversed polarity. The cytoplasm of the neoplastic cells was eosinophilic, with ovoid nuclei, fine chromatin, small nucleoli and occasional grooves and pseudo-inclusions. The mesenchyme of tumor was fibrous tissue with hyaline degeneration (Figure 5).
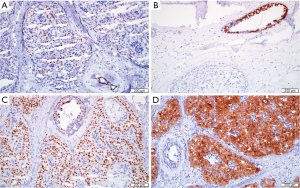
The results of IHC showed that SMMHC and p63 were negative staining, indicating the absence of myoepithelial cells. Focally positive expression of ER was found, with a rate of <1%. PR and HER-2 were negative staining. The Ki-67 proliferative index was low (2%). Tumor cells express diffuse positive in CK5 and Calretinin in the cytoplasm. Almost all tumor cells are positive for S0X-10 in the nucleus and GCDFP-15 was focally positive. Tumor cells nuclei were negative for TTF-1, but some normal duct epithelial cells around the tumor were positive for TTF-1. The tumor cells were focal positive for GATA-3 and negative for TG. Laminin around the tumor cell cluster was negative. EMA was negative staining (Figure 6A-6D). Molecular genetic analysis was conducted using WES, and the results showed concurrent IDH2 p.R172W and PIK3CA p.H1047R hotspot mutations (Table S1). Chromosomal aberrations were not analyzed. All of the results confirmed the diagnosis of TCCRP. The patient was alive and well for 33 months at the last follow-up.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The 2 cases we reported had typical pathological morphology of TCCRP, that is, solid and papillary structures, tall columnar tumor cells with nuclear groove and nuclear inclusion formation, similar to the high cell subtype of thyroid papillary carcinoma. Histologically, case 1 showed few cyst-like expansions in the ducts which was also seen in some spaces between fibrous vessel cores in case 2. Colella et al. reported a case of diffuse cystic dilatation of the duct, which appeared macroscopically as a special brownish translucent multinodular tissue (7). Case 2 was consistent with the typical TCCRP, demonstrated a well-circumscribed gray-white mass. Case 1 showed light red in color due to the blood on the surface. In addition, case 1 underwent core needle biopsy and intraoperative freezing pathology, but none of them could diagnose exactly. Since the core biopsy specimen showed most of the tumor cells neither had polar distribution or tall columnar character, it was easy to be misdiagnosed as papilloma (6,8,14). It may be related to tissue compression during the puncture process. The intraoperative freezing pathology may diagnose it as papillary lesions of the breast due to the extremely disordered distribution of tumor cells nuclei and the inability to determine the presence of myoepithelial cells, and TCCRP diagnosis might be delayed. These results indicated that the precise diagnosis of TCCRP required the combination of specific immunophenotype to avoid misdiagnosis.
IHC confirmed the diagnosis of TCCRP. The 2 cases in our group were all triple-negative phenotypes, CK5/6 was diffusely positive, and myoepithelial markers were negative, which were consistent with the phenotype of TCCRP in the literature previously reported (Table S2). GATA-3 were focally expressed in both cases of our group. Of the patients we evaluated from the previous cases, 42.9% were diffuse positive and 42.9% were focal expression. Studies have shown that as a marker for primary breast cancer, the sensitivity of GATA-3 is up to 94%, especially for ER-positive breast cancer. However, the sensitivity can reach only 43–66% to diagnose metastatic triple-negative breast cancer (TNBC). SOX-10 was diffusely expressed in our cases. The previous 75 cases of TCCRP were not tested for SOX-10. SOX-10 is mainly expressed in TNBC and metaplastic breast cancer, regulating the process of mesenchymal transition of breast epithelial cells. In addition, its expression in TNBC is significantly higher than other breast cancer subtypes, with a sensitivity of 69%. It is suggested that the simultaneous staining of GATA-3 and SOX-10 can improve the sensitivity of TCCRP to justify the origin of breast and distinguish from other carcinomas including metastatic thyroid carcinoma, etc. In our cases, positive expression of TTF-1 was found in few tumor cells and a small number of ductal epithelial cells at the periphery, while the negative rate of previous cases was 100%. TTF-1 is often expressed in thyroid cancer and lung adenocarcinoma, and is used to determine the primary site of metastatic cancer. However, it can also have varying degrees of positive expression in 2.5% of breast cancers, such as invasive ductal carcinoma, lobular carcinoma and other subtypes. The expression of TTF-1 in normal breast tissue needs to be further explored. TCCRP needs to be differentiated from metastatic PTC. The possibility of breast origin should not be denied because of focal TTF-1 expression, especially in core needle biopsy, and the results of breast-derived markers and TG staining should be comprehensively evaluated. It is worth noting that calretinin was positively expressed in previous case reports. Calretinin is mainly used for the diagnosis of mesothelioma, accounting for only 15% of breast cancers. Studies have shown that calretinin is often expressed in high-grade, basal-like phenotypes of breast cancer, with a positive rate of 67%. Our cases were also positive for calretinin. Calretinin is expected to become one of the auxiliary indicators of TCCRP diagnosis.
Our cases had hotspot mutations of IDH2 and PIK3CA. In the previous literature, 88% of patients had IDH2 mutations, 65% had PIK3CA mutations, and simultaneous mutations accounted for 60% (Table S2). IDH mutations are common in many tumors, such as acute myeloid leukemia, oligodendroglioma and chondrosarcoma, etc., resulting in abnormal epigenetic regulation, cell differentiation, and thus tumorigenesis. In 2016, Chiang et al. detected the IDH2 and PIK3CA mutations for the first time and proposed that they might be the driving factors of TCCRP and contacted them with the “reverse polarization” of tumor cells (3). In addition, IDH2 R172W mutation was detected in case 2, which was not belong to the common IDH2 single-nucleotide variants of TCCRP, namely R172S, R172G, R172T. Zhong et al. reported 1 case of IDH2 R172W mutation in the previous cases (13). Bhargava et al. and Alsadoun et al. indicated that some TCCRP cases did not have IDH2 mutations, but had mutations in genes such as PRUNE2, ATM, and KIT (8,10). Whether other mutations besides the hotspot mutations have indicative significance for TCCRP still needs more follow-up case reports to explore. Currently, IDH2 mutation is specific for the diagnosis of TCCRP, and the combination with clinicopathological features can clarify the diagnosis of TCCRP, which is conducive to the clinical treatment and prognosis evaluation. Additionally, Pareja et al. and Alsadoun et al. used monoclonal antibodies of IDH2 to perform IHC and compared that with the sequencing results. The results showed that IHC has good sensitivity and specificity for the detection of IDH2 mutation in TCCRP. It can be used as a diagnostic tool of TCCRP, particularly in biopsy and after resection (10,16). Relatively, PIK3CA mutation does not appear specific in TCCRP which indeed can also be found in various types of breast cancer including papilloma.
TCCRP should be distinguished from PTC and breast papillary lesions, including papilloma and papillary carcinoma. TCCRP is similar to PTC in histology, but lacks TG positive expression and RET gene rearrangement or BRAF gene mutation, excluding its thyroid origin (19). TCCRP is easy to be confused with breast papillary lesions as it shares papillary architecture with lack of myoepithelial cells. Nevertheless, TCCRP can be differentiated from these entities by the characteristic morphology of cells with reverse polarity and immuno-positivity for calretinin. The myoepithelial surrounding the infiltrating epitheliosis of the breast (IE) is intact, discontinuous or completely absent, and has similarity with TCCRP in terms of immunophenotype. Eberle et al. used massively parallel sequencing to detect related genes of IE, and the results showed that more than half of the cases had PIK3CA hotspot mutations (20). Therefore, the characteristic “reverse polarity” distribution of TCCRP, the positive expression of calretinin and the IDH2 mutation are still the main points of differentiation from IE.
Given the rarity and limited reports of TCCRP, there is no clear guidance regarding its treatment at present. TCCRP is generally considered to be a low-invasive tumor with indolent behavior. Patients mainly receive breast conserving surgery, including local extensive resection and segmental resection. No clear indications exist for lymph node dissection, radiotherapy and chemotherapy. Bhargava et al. treated a patient with contralateral invasive ductal carcinoma after neoadjuvant chemotherapy, but no significant change was found in TCCRP lesions (8). The average follow-up time of previous cases was 29 months (3–132 months). During the follow-up period, most patients were alive and well, with only 1 case of local recurrence and 1 case of bone metastasis. Cameselle-Teijeiro et al. reported the only case of bone metastasis, but due to poor quality of the picture, it was difficult to recognize its typical morphology to eliminate the diagnosis of ER-positive breast cancer (4).
In summary, TCCRP is an extremely rare invasive breast cancer with a solid papillary structure lined by tall columnar cells and “reverse polarity” nucleus distribution. However, the diagnosis of TCCRP by core needle biopsy can be challenging due to the atypical histochemical characteristics. Despite IDH2 and PIK3CA hotspot mutations, other gene mutations including PRUNE2 and ATM, etc. could also be found in TCCRP. Reviewing previous cases, we found that calretinin was positively expressed, which was expected to become one of the auxiliary diagnostic indicators in the future. Additionally, given the two cases we reported expressed TTF-1 focally in a few tumor cells and normal ductal epithelium cells, awareness should be taken during biopsy to avoid misdiagnosis. The diagnosis of TCCRP requires comprehensive analysis of pathological features, special immuno-phenotypes and gene mutations. Further research should be reported to characterize and deeply investigate the diagnosis and treatment criteria of TCCRP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-591
Peer Review File: Available at https://dx.doi.org/10.21037/gs-21-591
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-591). Xi-ru Li serves as an Editor-in-Chief of Gland Surgery from May 2017 to April 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eusebi V, Damiani S, Ellis IO, et al. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma: report of 5 cases. Am J Surg Pathol 2003;27:1114-8. [Crossref] [PubMed]
- Masood S, Davis C, Kubik MJ. Changing the term "breast tumor resembling the tall cell variant of papillary thyroid carcinoma" to "tall cell variant of papillary breast carcinoma". Adv Anat Pathol 2012;19:108-10. [Crossref] [PubMed]
- Chiang S, Weigelt B, Wen HC, et al. IDH2 Mutations Define a Unique Subtype of Breast Cancer with Altered Nuclear Polarity. Cancer Res 2016;76:7118-29. [Crossref] [PubMed]
- Cameselle-Teijeiro J, Abdulkader I, Barreiro-Morandeira F, et al. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma: a case report. Int J Surg Pathol 2006;14:79-84. [Crossref] [PubMed]
- Tosi AL, Ragazzi M, Asioli S, et al. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma: report of 4 cases with evidence of malignant potential. Int J Surg Pathol 2007;15:14-9. [Crossref] [PubMed]
- Chang SY, Fleiszer DM, Mesurolle B, et al. Breast tumor resembling the tall cell variant of papillary thyroid carcinoma. Breast J 2009;15:531-5. [Crossref] [PubMed]
- Colella R, Guerriero A, Giansanti M, et al. An additional case of breast tumor resembling the tall cell variant of papillary thyroid carcinoma. Int J Surg Pathol 2015;23:217-20. [Crossref] [PubMed]
- Bhargava R, Florea AV, Pelmus M, et al. Breast Tumor Resembling Tall Cell Variant of Papillary Thyroid Carcinoma: A Solid Papillary Neoplasm With Characteristic Immunohistochemical Profile and Few Recurrent Mutations. Am J Clin Pathol 2017;147:399-410. [Crossref] [PubMed]
- Foschini MP, Asioli S, Foreid S, et al. Solid Papillary Breast Carcinomas Resembling the Tall Cell Variant of Papillary Thyroid Neoplasms: A Unique Invasive Tumor With Indolent Behavior. Am J Surg Pathol 2017;41:887-95. [Crossref] [PubMed]
- Alsadoun N, MacGrogan G, Truntzer C, et al. Solid papillary carcinoma with reverse polarity of the breast harbors specific morphologic, immunohistochemical and molecular profile in comparison with other benign or malignant papillary lesions of the breast: a comparative study of 9 additional cases. Mod Pathol 2018;31:1367-80. [Crossref] [PubMed]
- Gai L, Done SJ, Cook D, et al. Breast tumour resembling tall cell variant of papillary thyroid carcinoma: case presentation (in a patient with Lynch syndrome). J Clin Pathol 2018;71:1031-2. [Crossref] [PubMed]
- Lozada JR, Basili T, Pareja F, et al. Solid papillary breast carcinomas resembling the tall cell variant of papillary thyroid neoplasms (solid papillary carcinomas with reverse polarity) harbour recurrent mutations affecting IDH2 and PIK3CA: a validation cohort. Histopathology 2018;73:339-44. [Crossref] [PubMed]
- Zhong E, Scognamiglio T, D'Alfonso T, et al. Breast Tumor Resembling the Tall Cell Variant of Papillary Thyroid Carcinoma: Molecular Characterization by Next-Generation Sequencing and Histopathological Comparison With Tall Cell Papillary Carcinoma of Thyroid. Int J Surg Pathol 2019;27:134-41. [Crossref] [PubMed]
- Haefliger S, Muenst S, Went P, et al. Tall cell carcinoma of the breast with reversed polarity (TCCRP) with mutations in the IDH2 and PIK3CA genes: a case report. Mol Biol Rep 2020;47:4917-21. [Crossref] [PubMed]
- Jassim M, Premalata CS, Okaly G, et al. Tall Cell Carcinoma with Reverse Polarity of Breast: Report of a Case with Unique Morphologic and Molecular Features. Turk Patoloji Derg 2021;37:183-8. [PubMed]
- Pareja F, da Silva EM, Frosina D, et al. Immunohistochemical analysis of IDH2 R172 hotspot mutations in breast papillary neoplasms: applications in the diagnosis of tall cell carcinoma with reverse polarity. Mod Pathol 2020;33:1056-64. [Crossref] [PubMed]
- Zhang X, Wu H, Wang Z, et al. Tall cell carcinoma of the breast with reverse polarity: case report with gene sequencing and literature review. Gland Surg 2021;10:837-43. [Crossref] [PubMed]
- Ding LM, Hu HX, Wang YJ, et al. Tall cell variant of papillary breast carcinoma: report of a case. Zhonghua Bing Li Xue Za Zhi 2019;48:815-7. [PubMed]
- Hameed O, Perry A, Banerjee R, et al. Papillary carcinoma of the breast lacks evidence of RET rearrangements despite morphological similarities to papillary thyroid carcinoma. Mod Pathol 2009;22:1236-42. [Crossref] [PubMed]
- Eberle CA, Piscuoglio S, Rakha EA, et al. Infiltrating epitheliosis of the breast: characterization of histological features, immunophenotype and genomic profile. Histopathology 2016;68:1030-9. [Crossref] [PubMed]



