
Intraoperative neurophysiologic monitoring prevented iatrogenic spinal cord injury during robotic-assisted transabdominal adrenalectomy: a case report
Introduction
Myelopathy is derived from the Greek word myelos, meaning “spinal cord”, and pathos for “disorder of”. Various disease processes can disrupt the spinal cord; however, cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction in individuals greater than 55 years of age. CSM is characterized by gradual onset with periods of progression (1).
The cause of CSM is not known but it has mechanical and vascular mechanisms contributing to its pathophysiology. Mechanical factors can be divided into static and dynamic components. Spondylosis, or degenerative changes occurring with age, disrupts the integrity of the vertebral column. Intervertebral discs dehydrate as they age, leading to collapse fragmentation (2). Because of disc degeneration, there are increased mechanical forces on the vertebral body endplates and subsequent osteophyte formation. Osteophytes stabilize adjacent vertebrae by increasing the weight-bearing surfaces of the endplates. However, osteophyte formation can decrease the diameter of the spinal canal, compressing the spinal cord (3). Spinal cord compression may also occur from disc herniation, ossification of the posterior longitudinal ligament, hypertrophy of the ligamentum flavum, and subluxation or kyphosis of the cervical spine. These factors have an even greater impact on patients with underlying congenital stenosis (3). Normal movement of the spinal cord may exacerbate injury from spinal stenosis. During flexion, the spinal cord stretches, which may stretch the spinal cord as it traverses new osteophytes, with cord compression and ischemia (2). The spinal cord may also compress during extension secondary to a hypertrophied ligamentum flavum pressuring the posterior columns. Mechanical factors cause local deformity and ultimately reduced arterial blood flow. The vascular factor is supported by observing that the C5 to C7 spine not only has the highest frequency of CSM but is also the area of the spinal cord with the most tenuous vascular supply (3).
The benefit of intraoperative neurophysiologic monitoring (IONM) with somatosensory evoked potentials (SSEPs) and transcranial motor evoked potentials (TcMEPs) has been documented in the spine, intracranial, thyroid, vascular, and orthopedic surgeries as a way to avoid an iatrogenic nerve injury (4-6), but not in specialized intraabdominal general surgery. SSEPs monitor the function of the ascending sensory dorsal column-medial lemniscus pathway of the spinal cord, usually by stimulating the upper extremity ulnar nerve and the lower extremity posterior tibial nerve. A decrease in SSEP amplitude of more than 50% or a combined increase in SSEP latency of more than 10% of the baseline is generally accepted as the “alarm criteria” for potential intraoperative neural damage (7). TcMEPs monitor the function of the descending motor corticospinal tracts by recording the corresponding activated myotome. There is variation in which objective findings in TcMEPs are generally accepted as “alarm criteria” for potential intraoperative neural damage (8).
The laparoscopic transabdominal approach in the lateral decubitus position, which was first reported in 1992, is now the standard for adrenalectomies, for both hormone-secreting and non-secreting, small to medium-sized (≤6 cm) benign adrenal tumors (9,10). One of the main advantages of the transabdominal lateral approach is the gravity-facilitated exposure of the adrenals (9). However, the lateral decubitus position is associated with a high incidence of position-related SSEP changes. The incidence of SSEP changes from the upper extremity while in the lateral decubitus position is 7.5% (11). While compression is known to be the leading etiology of impending peripheral nerve injury in the lateral decubitus position, the incidence of iatrogenic spinal cord injury (SCI) in the lateral decubitus position is unreported.
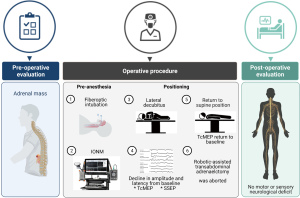
We report the first case of IONM during a robotic-assisted transabdominal left adrenalectomy requiring right lateral decubitus positioning (Figure 1). While the patient had no formal diagnosis of CSM, underlying myelopathy was suspected based on a thorough history and focused neurological exam in the setting of known cervical stenosis. The application of IONM was utilized to prevent an iatrogenic SCI, with the potential for paralysis. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-235).
Case presentation
A 74-year-old male with a left adrenal mass presented for robotic-assisted transabdominal adrenalectomy. His past medical history included hypertension, diabetes mellitus type II, chronic kidney disease stage III, idiopathic colitis, two parastomal hernia repairs, and multiple abdominal surgeries, including ileostomy and laparoscopic cholecystectomy, as well as what was proven later as cervical spinal stenosis with radiculopathy. The patient had a 3 cm mass when first discovered by abdominal CT scan more than one year before the scheduled adrenalectomy. Prior to surgery, the mass increased from 3 to 4.5 cm but remained stable in size at 4.5 cm. The Hounsfield unit determination was −1.6, corresponding to a high likelihood of being a benign adrenal adenoma. However, the patient endorsed symptoms of early satiety leading to weight loss and chronic left-sided-back pain stemming from the mass.
During the preoperative evaluation, decreased neck extension with associated pain and bilateral upper extremity radiculopathy was identified. A focused neurological exam revealed 5/5 motor strength to all extremities, +2 DTRs throughout, and negative Hoffman’s sign bilaterally. Recent spinal imaging was not available. When positioned supine on the operating room table, the patient described upper and lower extremity neurological symptoms, low back pain with “electrical impulses” radiating into his bilateral lower extremities indicating upper motor neuron lesion. These findings resolved once the patient was ramped with sheets to maintain him in a flexed position. Concern developed regarding the maintenance of adequate flexion after turning the patient into the lateral decubitus position. The existence of Lhermitte’s sign, bilateral upper extremity pain was suggestive of CSM. However, the patient did not have other symptoms such as bilateral upper and lower extremities weakness, hyperreflexia, unsteady gait. The patient also has no symptoms to support lower motor neuron injury with normal DTR, motor strength, and normal sensory examination.
The decision was made to proceed with surgery because of the patient’s progressive symptoms and since he had traveled a long distance to have surgery in our institution. To minimize possible SCI, the induction of general anesthesia and airway management using an awake fiberoptic intubation and the use of IONM with SSEP and TcMEP were selected.
The neurophysiological monitoring protocol used commercially available neurophysiological monitoring workstations (Axon EpochXP), with the bilateral and multimodal recording of evoked potentials as described by Schwartz et al. (12). TcMEP myogenic responses were recorded bilaterally from the first dorsal interosseous muscles in the upper extremities and the abductor hallucis muscles in the lower extremities after eliciting the transcranial electrical stimulator (Digitimer D185) that delivered a brief (50-µsec), high-voltage (250 to 500-V) signal between two corkscrew electrodes inserted subcutaneously over motor cortex regions C1-C2 (International 10-20 System) (13,14). Cortical and subcortical SSEPs were elicited by 500-µVolt electrical pulses to the bilateral posterior tibial and ulnar nerves at 15 ms intervals and were recorded from either the gold-plated cup or subdermal needle electrodes affixed to Cpz, Cp3, and Cp4 and referenced to Fpz (International 10-20 System) (13,14). Subcortical responses were recorded similarly with electrodes placed over the surface of the second or third cervical vertebra and also referenced to Fpz.
Immediately following awake fiberoptic intubation prior to induction of anesthesia, complete neurological evaluation was performed by anesthesiologist that indicated muscle strength 5/5 with no motor deficit bilaterally, deep tendon reflexes +2 at biceps and patellar tendons. No focal deficit was noted on examination as this time. Prior to administration of anesthesia, the patient was vitally stable, pulse 90 bpm, Blood Pressure of 110/73 mmHg, and temperature 98.6 F. The patient received 20 µg of IV dexmedetomidine for sedation while topical anesthesia of the airway preparation included 5 mL of 4% nebulized lidocaine to block the sensory innervation of the vocal cords and trachea. Subsequently, glossopharyngeal nerve blocks were performed by coating the tonsillar pillars in 2% lidocaine gel cotton swabs. Superior laryngeal nerve blocks were performed with a 25-gauge needle where 2 mL of 2% lidocaine was deposited bilaterally. Awake fiberoptic intubation was performed without incident and manipulation of the patient’s cervical spine. Baseline amplitude and latencies for TcMEPs and SSEPs were established in the supine position before repositioning the patient into the right lateral decubitus position. At this point, the patient was vitally stable with no significant change from the baseline. The supine baselines showed large-amplitude TcMEPs from the myotome at the right ankle (Figure 2A), and large-amplitude SSEPs from the cortex upon the right posterior tibial nerve stimulation (Figure 2B). Vital signs continued to be the same following induction of anesthesia.
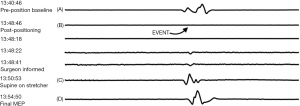
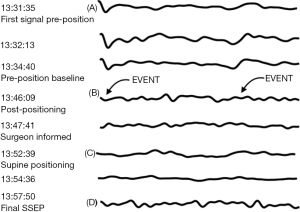
Approximately five minutes after assuming the right lateral decubitus positioning of the patient and flexion of the operating room table, IONM demonstrated loss of TcMEPs in the right ankle myotome and markedly attenuated SSEP signals from right popliteal nerve stimulation. The surgical team was immediately notified of the attenuated signals, the procedure was aborted, and the patient was returned to the supine position on the operating table. Within minutes of repositioning the patient supine, the TcMEP from his right ankle myotome returned to its baseline, pre-position amplitude (Figure 2C), which was again confirmed with the final TcMEP reading before the patient left the operating room (Figure 2D). The SSEP from his right posterior tibial nerve, however, did not return to baseline, pre-position amplitudes, and remained attenuated in the supine position (Figure 3A-3C). The final SSEPs recorded from the patient were still attenuated (Figure 3D). However, he exhibited normal extremity movement, strength, and sensation before leaving the operating room. The concomitant association of incidence of upper and lower extremity neurologic symptoms bilaterally with perioperative cervical spine manipulation was suggestive of cervical spinal cord lesion. Differential diagnosis included CSM, multiple sclerosis, amyotrophic lateral sclerosis as well as neck masses. However, other differential diagnosis such as multiple sclerosis, amyotrophic lateral sclerosis and masses (metastatic tumors) that press on the spinal cord were unlikely given the patient age, and circumstances with onset related to neck manipulation.
In the post-anesthesia care unit, the patient was again evaluated after full emergence from all anesthetics and continued to demonstrate normal extremity movement and sensation without residual neurologic deficits. Neurosurgery was consulted for evaluation and management, and a postoperative cervical, thoracic, and lumbar spine MRI without contrast was obtained. Of note, the patient experienced great difficulty laying completely supine for the imagining to be obtained and, ultimately, required a MAC anesthetic. Imaging revealed significant spinal stenosis at C2-C3, C3-C4, C4-C5, and C5-C6 levels. Neurosurgery performed a C2-T1 spinal fusion with C3-C6 laminectomies the following day. The patient fully recovered without any deficits from the neurosurgical standpoint.
All procedures detailed in this case report were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
CSM is the most common cause of spinal cord dysfunction in patients older than 55 years of age. The diagnosis of CSM is often delayed or not made due to the insidious course of the disease process, asymptomatic presentation, and lack of pathognomonic findings. In a retrospective study of 42 patients who ultimately underwent surgery for their CSM, an average of 5.2 consultations were required before the definitive diagnosis was made; the delay from onset of symptoms to diagnosis was deemed to be 2.2 years (15). This comorbidity becomes more prevalent as the patient population ages; in the asymptomatic male and female population older than 60–65 years of age, approximately 95% of men and 70% of women showed radiologic spondylotic changes (16).
Myelopathic patients are at an increased risk of experiencing an iatrogenic SCI during an elective, non-spinal surgery such as tetraplegia or quadriplegia, a rare but devastating complication. There are 18 reported cases of tetraplegia following elective, non-spinal surgery in patients with undiagnosed CSM. Of these patients, only 16% were known to have cervical stenosis (14). Furthermore, only nine patients were symptomatic, and 16 patients had normal neurological exams. Our patient illustrates the need for a high degree of suspicion for undiagnosed CSM and to formulate an unprecedented anesthetic plan to avoid adverse outcomes.
A known risk factor for CSM is a congenitally narrow cervical spinal canal. While there is no well-defined pattern of neurologic deficits, alterations in balance and gait impairment are possible. Patients may report episodes of recent falls or new use of a cane while ambulating. Patients describe some degree of either sensation disturbance, upper extremity numbness or decreased vibratory sense, or loss of fine motor function of the hands by the time of surgical presentation. Neck pain is also common in these patients and is often a reason they seek treatment. On neurological exam, signs of upper motor neuron lesion may be present, such as a positive Hoffman’s sign or hyperreflexia. Additionally, the sensation of upper extremities, grip strength, and an attempt to elicit a Lhermitte sign should be performed. If available, MRI should be reviewed as this imaging modality identifies spinal cord inflammation, edema, lesions, and high signal changes (17).
IONM is a technique frequently employed in spinal surgery, intracranial procedures, thyroid surgery, and some vascular procedures (4-6). However, the use of IONM with SSEP and TcMEP to monitor and prevent impending iatrogenic SCI has not yet been documented in abdominal surgeries, such as in laparoscopic or robotic-assisted transabdominal adrenalectomies requiring lateral decubitus positioning. SSEPs and TcMEPs have a high specificity indicating that normal IONM findings correlate highly with an uninjured spinal cord when used in combination. For this precise reason, IONM was incorporated into the anesthetic approach of this patient with a known past medical history of cervical stenosis presenting for an elective robotic-assisted transabdominal adrenalectomy requiring the right lateral decubitus position. IONM detected transient loss of SSEPs and TcMEPs in the right lower extremities shortly after positioning the patient into the right lateral decubitus position, at which point the procedure was aborted and the patient was immediately returned to the supine position. The TcMEPs quickly returned to baseline values within minutes, while the SSEPs remained attenuated. However, the patient did not exhibit loss of strength, motion, or sensation in bilateral upper and lower extremities postoperatively. Thus, postoperative iatrogenic SCI was successfully prevented with the use of IONM by detecting attenuations and loss of signal in both SSEPs and TcMEPs from the right lower extremities. If the case proceeded without utilizing IONM, the patient would likely have suffered an iatrogenic SCI, with the potential for paralysis.
Improvement of the intraoperative motor evoked potential has been associated with a better postsurgical outcome in CSM (18). Lo et al. showed that motor evoked potential improvement is observed in a much larger proportion of cervical decompression surgery cases than previously thought (18).
The spinal dysfunction and cord injury seen in CSM patients under general anesthesia is multifactorial and incompletely understood. While both mechanical and vascular components have been identified as contributing factors, the role of relative hypotension (19) and patient positioning, such as lateral decubitus (11), have been investigated. This case demonstrates the benefit of using IONM in non-spinal surgeries as the monitoring overcomes the incomplete understanding of the perioperative decline by identifying neurologic decline in real-time. This case also shows the importance of a low threshold for IONM as a standard of care for patients with a high likelihood of underlying undiagnosed CSM in addition to the established protective measures taken intraoperatively for myelopathic patients.
We suggest that a detailed preoperative screening for CSM with history and focused neurological exam be instituted as the standard of care during preoperative assessment prior to a non-elective spinal surgery in the at-risk population. Furthermore, referral to a spine surgeon for risk-benefit stratification and preoperative MRI, with possible neurosurgical intervention, maybe warranted before proceeding with elective, non-spinal surgery. CSM is treatable, with spinal decompression as the current gold standard. If the urgency or socio-economic restrictions of the non-spinal surgery do not allow for the preoperative screening for undiagnosed CSM or in case of the patient rejection of neurosurgical consultation and/or intervention, we suggest implementing IONM as a standard of care for at-risk patients in addition to the established myelopathic precautions.
Acknowledgments
A sincere thank you to Loula Burton from Tulane’s Research Proposal Development Office for her diligent editing and proofreading of this paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-235
Peer Review File: Available at https://dx.doi.org/10.21037/gs-21-235
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-235). EK serves as the Editor-in-Chief of Gland Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holly LT, Moftakhar P, Khoo LT, et al. Surgical outcomes of elderly patients with cervical spondylotic myelopathy. Surg Neurol 2008;69:233-40. [Crossref] [PubMed]
- Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976) 1998;23:2730-7. [Crossref] [PubMed]
- Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: pathophysiology, natural history, and clinical evaluation. Instr Course Lect 2003;52:479-88. [PubMed]
- Cunningham JN Jr, Laschinger JC, Merkin HA, et al. Measurement of spinal cord ischemia during operations upon the thoracic aorta: initial clinical experience. Ann Surg 1982;196:285-96. [Crossref] [PubMed]
- Huang S, Garstka ME, Murcy MA, et al. Somatosensory evoked potential: Preventing brachial plexus injury in transaxillary robotic surgery. Laryngoscope 2019;129:2663-8. [Crossref] [PubMed]
- Lall RR, Lall RR, Hauptman JS, et al. Intraoperative neurophysiological monitoring in spine surgery: indications, efficacy, and role of the preoperative checklist. Neurosurg Focus 2012;33:E10 [Crossref] [PubMed]
- Halpern DK, Liu HH, Howell RS, et al. Neural Monitoring for Robotic Abdominal Wall Reconstruction. JSLS 2020;24:e2020 [Crossref] [PubMed]
- Tsutsui S, Yamada H. Basic Principles and Recent Trends of Transcranial Motor Evoked Potentials in Intraoperative Neurophysiologic Monitoring. Neurol Med Chir (Tokyo) 2016;56:451-6. [Crossref] [PubMed]
- Raffaelli M, De Crea C, Bellantone R. Laparoscopic adrenalectomy. Gland Surg 2019;8:S41-52. [Crossref] [PubMed]
- Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Kamel I, Zhao H, Koch SA, et al. The Use of Somatosensory Evoked Potentials to Determine the Relationship Between Intraoperative Arterial Blood Pressure and Intraoperative Upper Extremity Position-Related Neurapraxia in the Prone Surrender Position During Spine Surgery: A Retrospective Analysis. Anesth Analg 2016;122:1423-33. [Crossref] [PubMed]
- Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am 2007;89:2440-9. [Crossref] [PubMed]
- Herwig U, Satrapi P, Schönfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 2003;16:95-9. [Crossref] [PubMed]
- Kamel I, Barnette R. Positioning patients for spine surgery: Avoiding uncommon position-related complications. World J Orthop 2014;5:425-43. [Crossref] [PubMed]
- Behrbalk E, Salame K, Regev GJ, et al. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus 2013;35:E1 [Crossref] [PubMed]
- Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine (Phila Pa 1976) 1986;11:521-4. [Crossref] [PubMed]
- Toledano M, Bartleson JD. Cervical spondylotic myelopathy. Neurol Clin 2013;31:287-305. [Crossref] [PubMed]
- Lo YL, Zhu L, Soh RC, et al. Intraoperative Motor Evoked Potential Improvement in Cervical Spondylotic Myelopathy: Comparison of Cortical Stimulation Parameters. J Clin Neurol 2020;16:102-7. [Crossref] [PubMed]
- Kim KA, Wang MY. Anesthetic considerations in the treatment of cervical myelopathy. Spine J 2006;6:207S-211S. [Crossref] [PubMed]


