
Use of targeted therapy and immunotherapy for the treatment of yolk sac tumors in extragonadal pelvic sites: two case reports
Introduction
Yolk sac tumor (YST), also known as endodermal sinus tumor, is a highly malignant germ cell tumor arising from primordial germ cells. About 80% of YSTs are found in the gonads (ovaries, testes), and 10–20% are of extragonadal origin (EGYST) (1). Primordial germ cells or pluripotent embryonic stem cells grow into the extraembryonic mesoderm and endoderm, forming a yolk sac-like structure. During the migration of the yolk sac to the genital ridge, some germ cells may be lodged in extragonadal sites, and differentiate into a YST that is usually found in the midline of the body (2). Accounting for about 4–7% of ovarian malignant tumors, YSTs originating from the ovary mainly involve adolescents and young women, with a poor prognosis. Those arising from outside the ovary are even rarer, with only 26 cases reported worldwide yet, including just four non-endodermal cases (3-5). A YST arising from the cervix has been reported in a 10-month-old infant (6). The clinical diagnosis of EGYST is a great challenge, usually relying on postoperative pathological examination. Uterine YST is confirmed with pathological tumor-specific features observed under a light microscope, including Schiller-Duval (SD) bodies, reticular formation, hyaline bodies, positive expressions of alpha-fetoprotein (AFP), pan cytokeratin (CK-pan), and local positive expression of carcinoembryonic antigen (CEA). Sal-like protein 4 (SALL4) is an immunomarker with good specificity for the diagnosis of YST. Its positive rate and positive intensity are superior to AFP and Glypican-3, and it is especially helpful for the differentiation of SALL4 from ovarian granuloblastoma and clear cell carcinoma. All these features have been found in the four reported cases of YSTs developing from myometrium and serosal layers. A combination of maximum surgical resection and chemotherapy/radiotherapy is preferred for uterine or ovarian YST. Chemotherapy based on bleomycin, etoposide, and cisplatin (BEP) is recommended as the standard regimen. However, the overall survival of non-endodermal YST is poor, with only 14 months in recurrent cases (Table 1).
Table 1
| Case | Age (years) | AFP (ng/mL) | Tumor location and tumor size (cm) | Surgical option | Pathological characteristics | Metastatic sites | Chemotherapy regimen | Radiotherapy | Recurrent location and time of recurrence | Overall survival (month) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | 34,118 | Middle and lower anterior wall of the uterus, 14 cm | A | SD; AFP (+) | Peritoneum, omentum majus, liver | Cisplatin bleomycin | N/A | No | 72 |
| 2 | 28 | 26,500 | Myometrium anterior wall of the uterus | A | SD; AFP (+) | Peritoneum, and omentum majus | Vincristine dactinomycin cyclophosphamide, etoposide cisplatin | N/A | Pelvic cavity | 14 |
| 3 | 39 | Not detected | Between the lower uterine segment and the posterior wall of bladder | B + right ileum and colon | SD; AFP (+) | Ileum, and colon | Vincristine dactinomycin cyclophosphamide | N/A | No | 102 |
| 4 | 30 | 5,765 | Myometrium | C | SD; AFP (+) | None | Bleomycin etoposide cisplatin | N/A | No | 12 |
| 5 | 18 | 1,210 | Myometrium of posterior wall of uterus, 10 cm | C + right ureteral stent implantation | SD; AFP (+) | None | Nidaplatin etoposide, bleomycin etoposide nidaplatin, paclitaxel cisplatin, ifosfamide etoposide | N/A | Vaginal stump, right pelvic cavity, left supraclavicular lymph node, at 9 months postoperatively | 35 |
| 6 | 32 | 20,251 | Between the lower anterior wall of the uterus and the bladder, 8 cm | C | SD; AFP (+) | Bladder, omentum majus and peritoneum | Etoposide bleomycin nedaplatin, paclitaxel liposome nedaplatin bevacizumab | N/A | Pelvic cavity | 21 (undergoing chemotherapy and immune therapy) |
A: resection of the uterine, omental, and peritoneal metastases; B: resection of the uterine, bilateral fallopian tubes and ovaries, omentum majus; C: resection of the myometrium, pelvic paraaortic lymph nodes and omentum majus + uterine reconstruction. EGYST, extragonadal yolk sac tumor; AFP, alpha-fetoprotein; SD, Schiller-Duval; N/A, not applicable.
The present two cases of YSTs of primary myometrium and serosal layer origins in were the 5th and 6th cases reported in the world. These patients were contacted by telephone to obtain verbal informed consent. They were neither metastases from ovarian cancer, nor primary cancer of myometrium origin. Case 1 died of recurrence and metastasis after a combination of surgery and chemotherapy. Case 2 experienced relapse and was treated with targeted therapy and immunotherapy, a regimen that had never been reported before but prolongated her survival. We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-663).
Case presentation
Case 1
A female, 18 years old, was admitted to the hospital due to “lower abdominal pain for a week” on 22 November 2013. The patient had no history of ovarian cancer in her family for three generations. A B-ultrasound showed huge uterine fibroids (10 cm ×9 cm ×8 cm). Preoperative AFP was 1,210 ng/mL. Intraoperative observation on 26 November 2013 showed that the tumor had a size of about 10 cm ×9 cm ×9 cm, an unclear boundary, and poor mobility. The blood vessels on the tumor surface were distended. The tumor was brittle, bled easily when touched, and densely adhered to the right pelvic wall, rectal and uterine depression, and part of the mesentery. No obvious abnormality was observed in the appearance of bilateral fallopian tubes. Cystic change was observed in both ovaries and a little bloody effusion appeared in the pelvic cavity. A small amount of tumor tissue was removed and sent for rapid frozen section analysis, which revealed a uterine malignant tumor. After communicating with the patient’s family, the approach was switched to open surgery. The final procedure was “total abdominal hysterectomy + tumor cytoreduction surgery + right pelvic lymphadenectomy + right partial ovarian resection + bilateral ovarian transposition + right ureteral stent placement”. The patient presented at International Federation of Gynecology and Obstetrics (FIGO) stage IV.
Postoperative pathological examination revealed the pelvic tumor tissue in the form of a mesh, with SD-like bodies. The tumor cells were cubic, columnar, or flat, and the cytoplasm was transparent or eosinophilic. Nucleoli, mitotics, and hyaline bodies were found in the focal area and hemorrhagic necrosis. Cancer cells were detected in ascites. The results of immunohistochemistry (IHC) were as follows: AFP positive (+), SALL4 (+), CK-pan positive (+). The following features were also distinguished: placental alkaline phosphatase (PLAP): focal area positive (+); human chorionic gonadotropin (HCG): local positive (+); CEA: focal area positive (+); cancer antigen 125 (CA125): focal area weakly positive (+); Ki-67: 50% positive (+); prostate-specific antigen (PSA): focal area positive (+); and estrogen receptor (ER)/progesterone receptor (PR): negative (−). In combination with hematoxylin and eosin (HE) staining and IHC results, the diagnosis was pelvic YST on the myometrium and serosal layers. Recidivism was seen on the lower resection margin and the deep layer of the cervical fibromuscular wall. The endometrium showed proliferative changes. The right ovarian cystic follicle and the right obturator lymph node displayed no metastases (Figure 1).
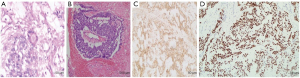
Postoperative adjuvant treatments
Intraperitoneal chemotherapy (nedaplatin + VP16) was initiated from postoperative D9; intravenous chemotherapy (BLM + VP16 + nedaplatin) started from postoperative D22. After three rounds of intravenous and intraperitoneal chemotherapy, only intravenous chemotherapy was performed with an interval of 3 weeks, for six cycles in total. During the 4th chemotherapy, the AFP was within the normal range on 20 September 2014. At 3 months after the chemotherapy, AFP had risen to 28.69 ng/mL. Positron emission tomography/computed tomography (PET-CT) revealed left supraclavicular lymph node metastasis. The chemotherapy regimen was changed to docetaxel plus cisplatin (TP) for four rounds. The AFP was 12.6 ng/mL after chemotherapy. A PET-CT scan performed at Fudan University Affiliated Cancer Hospital (Shanghai) showed tumor recurrence in the vaginal stump, metastatic nodules on the right side of the pelvis, and metastatic lymph nodes on the left supraclavicular. The chemotherapy regimen was changed to “ifosfamide + etoposide” for two rounds. The AFP rose to 168.1 ng/mL on 13 May 2015. Renal insufficiency and right hydronephrosis followed. The patient was lost to follow up and died on 14 November 2016.
Case 2
A 32-year-old female was admitted to the hospital on 15 March 2019 due to “lower abdominal pain for 2 weeks”. She had given birth to a boy before 3 years. A CT scan showed low-density shadow on the right of the appendix with a size of about 8.8 mm ×5.8 mm, raising suspicion of a malignant tumor. Her AFP was 20,251.0 ng/mL, and CA125 was 85.96 U/mL. In surgical exploration, an 8 cm mass in the lower right anterior wall of the uterus was found to have invaded the back wall of the bladder. The tumor was densely adhered to the peritoneum and greater omentum. The tumor tissue was extremely fragile, dark yellow, and had the texture of rotten fish. No obvious abnormal shape and size was observed in the bilateral ovaries and fallopian tubes, although two enlarged lymph nodes were found in the left pelvic cavity. The lymph node adjacent to the abdominal aorta was not significantly enlarged. The large omental tissue showed a brittle lump with a diameter of 3 cm. Dark yellow rotten fish-like tissue was collected for rapid frozen section analysis which revealed a malignant uterine tumor. Germ cells were considered as the tumor source. The uterus, bilateral fallopian tubes, bilateral ovaries, omentum, appendix, and pelvic lymph nodes were surgically resected. On account of the tumor having invaded the posterior wall of the bladder, quarter of the bladder was removed. The patient presented at FIGO stage IV.
Postoperative pathological examination showed that the uterine tumor tissue was sparsely reticulated and adenoid. The tumor cell nucleus was large and deeply stained, with aberrant, mitotic activity. Some tumor cells demonstrated transparent cytoplasm, local SD bodies, degenerated interstitial mucus. The results of IHC were as follows: AFP positive (3+), SALL4 (+), CK-pan positive (3+). The following features were also distinguished: PLAP: scattered positive (1+); HCG: negative (−); P53: positive (1+); PSA: positive (1+); and ER/PR: negative (−). Considering the HE staining and IHC results, the diagnosis was defined as uterine serosal surface YST infiltrating the fibromuscular wall, but not the mucosal surface of the cervical canal. There was no residual tumor tissue at the margin, and no tumor recidivism in the appendix. No tumor metastasis was found in retroperitoneal lymph nodes. The endometrium showed proliferative changes. Follicular cysts were seen in the left ovary. No abnormalities were found in the right ovary and bilateral fallopian tubes (Figure 2).
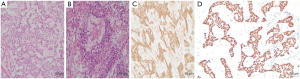
Postoperative adjuvant treatments
On 25 April 2019 (postoperative D37), intravenous chemotherapy (etoposide + BLM + nedaplatin) was initiated, with a frequency of once every 3 weeks. The AFP decreased to normal on the third dose. Intravenous combined with intraperitoneal chemotherapy was performed for three rounds, followed by intravenous chemotherapy for three rounds, with six cycles in total. At 3 months after chemotherapy, AFP increased to 5,120 ng/mL. A CT scan showed dilatation in the right renal pelvis and ureter, pelvic lymphadenopathy of the right iliac artery, and multiple pelvic metastasis. The chemotherapy regimen was changed to “paclitaxel liposome + nedaplatin + bevacizumab” on 3 April 2020. Following six cycles of chemotherapy, AFP decreased to 329 ng/mL; however, this increased to 19,544 ng/mL 2 months after the chemotherapy course. On 29 October 2020, the chemotherapy regimen was changed to “liposomal paclitaxel + nedaplatin + tislelizumab”. The AFP decreased to 13,297 ng/mL after two cycles of chemotherapy, and the patient is currently undergoing chemotherapy.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Approval was obtained from the ethics committee of Wuxi Maternal and Child Health Care Hospital Ethics Committee (document number: 2021-01-0202-04). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Clinical manifestations and diagnosis of YST in extragonadal pelvic site
In the early phase of embryonic development, the embryo complex is composed of blastoderm, amniotic sac, and yolk sac. The yolk sac is located outside the amniotic sac and connected to the fetus through the yolk tube. The YST is the commonest malignant germ cell tumor, mainly found in the young with an average age of 14 years, but also occasionally in postmenopausal women. EGYST often occurs in the posterior peritoneum, vagina, sacrococcyx, omentum, pineal gland, third ventricle, liver, and heart (7,8). EGYST of non-endometrial origin is even rarer, with only four cases reported all over the world. Only one case reported in China was a 1.5-year-old girl, and the rest have all been adult women (5) who have shown irregular vaginal bleeding, abdominal mass, pain, and so on.
EGYST may arise from the abnormal differentiation of human cells. The germ cells appear in the yolk sac near the allantois in the early stage (21 days of human embryo), move along the yolk sac wall and the posterior mesentery, pass the gonadal ridge, and enter the genital ridge at the 6th week to participate in primordial reproduction. In the genital ridge, these germ cells are blocked in the midline appendages of the posterior wall. If they do not degenerate but continue to grow and differentiate, they may grow into YSTs. This is the second possible pathogenesis of YST. The disease has a rapid course with focal necrosis and hemorrhage, and is brittle and highly malignant. The YST progresses through local infiltration, lymphatic metastasis, and blood metastasis, characterized with microcysts, endodermal sinusoids, solid, duct-actinoid, and reticulated structure (9,10). Because YST is often mixed with germ cell tumor components, it is necessary to exclude the possibility of germ cell tumor.
Often, YST is mixed with components of germ cell tumor. Therefore, germ cell tumor should be carefully excluded by microscopic observation. In addition, the morphology and immunophenotype of YST are similar to those of clear cell carcinoma, endometrioid adenocarcinoma, high-grade serous tumors, and other epithelial malignant tumors, leading to a challenge in the pathological diagnosis (11). Elevated serum AFP is a vital biological characteristic of EGYST and SALL4 can be used for pathological diagnosis of germ cell tumors. A high level of AFP was detected in the serum, cerebrospinal fluid, and ascites of these two cases, and was significantly reduced after tumor resection. As a result, preoperative AFP can be used to assist the clinical diagnosis, and the postoperative AFP serves as a biomarker for monitoring recurrence and predicting prognosis. The IHC staining of AFP and SALL4 are usually strongly positive, and can be used for differential diagnosis of cases with atypical histological characteristics under a microscope. Notably, the possibility of primary ovarian tumor should be ruled out. Here we reported two cases of YST of uterine origin. We did not detect abnormalities in the ovaries, and the tumors did not involve the endometrium. Postoperative pathological examinations showed benign lesions in bilateral ovaries in one case, and normal ovaries in the other. Moreover, the endometrium showed proliferative changes in both cases. We therefore made a diagnosis of primary YST originating from the myometrium and serosa, which was extremely rare. A significant elevation and strongly positive IHC staining of AFP were detected in both cases. Typical SD bodies observed under the electron microscope further supported the diagnosis. Therefore, clinical and pathological characteristics of rare non-endometrial EGYST are similar to those of previously reported YSTs of the ovary. Clinical and pathological characteristics of the two cases and previously published four cases of YSTs are summarized in Table 1.
Treatment and follow-up of EGYST
Due to its rare occurrence, there has been no systematic study on the treatment of EGYST in the pelvic cavity. For ovarian YST, treatment options include cytoreductive surgery and postoperative chemotherapy. Due to tumor infiltration and distant metastasis to surrounding organs and tissues, most of the patients are difficult to complete resection, poor surgical efficacy, and poor overall survival. There is controversy about whether surgery can preserve the endocrine function of young women and whether the greater omentum should be removed. In the published literature, eight cases of EGYST underwent resection of fallopian tubes and ovaries, all losing endocrine function. However, there have also been two cases reported of partial ovarian retention, including a 29-year-old woman with her right ovarian appendage preserved and a follow-up of 39 months without any recurrence (12); for the other 30-year-old women with endometrial YST, only the uterus was removed and bilateral ovarian appendages were retained, with a 6-year follow-up without any recurrence (13). Simpson et al. showed that comprehensive staging and cytoreductive surgery were of decisive significance for the survival and prognosis of patients (14). Therefore, it may be safe to consider a comprehensive staging operation that preserves endocrine function for young women under 40. A positive staining of AFP was detected in the inferior uterine margin of Case 1, which may have been the main reason for the recurrence of vaginal stump, in which case a postoperative adjuvant vaginal brachytherapy should be recommended. In addition, normal ovaries were preserved in this young case, which may bring risks of postoperative local or distant metastasis. The safety of endocrine surgery with ovarian preservation, as a result, requires a further validation.
Due to its lack of effective treatment methods and insensitivity to radiotherapy, the prognosis of EGYST was once extremely poor. New chemotherapy regimens have significantly improved this prognosis. Chemotherapy based on BEP is recommended as the standard regimen, similar to that for ovarian YST. Chemotherapy should be timely, adequate, and standardized. Insufficient chemotherapy can easily lead to tumor recurrence. The most effective measure to improve survival is surgery. Saltzman et al. (6) reported a 10-month-old infant with a cervical YST. In this case, the AFP level before chemotherapy was 18,592 ng/mL, but dropped to 11 ng/mL after four cycles of BEP chemotherapy before surgery. A CT scan showed that the tumor burden was reduced by 90%. After the 5th BEP treatment, surgery was performed because the tumor did not change any longer. The prognosis was good.
Notably, intravenous combined with intraperitoneal chemotherapy was performed in Case 2 because of extensive abdominal metastasis, which was also the potential reason for the short-term recurrence. Through literature review, scant evidence was found on the targeted therapy of recurrent YST. In this case, a combination of platinum-based chemotherapy and targeted antiangiogenic drugs (bevacizumab) was performed for postoperative recurrence. After six cycles of chemotherapy, AFP level significantly decreased, then sharply elevated in a short period. The patient was then treated with chemotherapy combined with immune therapy, and a decline of AFP level was observed. It is concluded that chemotherapy combined with targeted antiangiogenic drugs and immune therapy may be optimal to increase the survival of patients with advanced recurrent non-endometrial YST. Nevertheless, a detection of immune biomarkers is necessary for assessing the therapeutic efficacy.
There are some limitations to these case reports. First, the second patient was given chemotherapy and combined targeted and immunotherapy after surgery, referring to the current comprehensive treatment plan for epithelial ovarian cancer. However, target detection was not performed before treatment, so it was difficult to evaluate whether the survival benefit of the patient was brought by targeted drugs. Second, the follow-up time of patients was short, and further follow-up is needed to clarify the drug efficacy.
In conclusion, extragonadal pelvic YST is extremely rare, in contrast to the non-endometrial YSTs arising from the ovary. According to previously reported cases and our experiences, therapeutic strategies of extragonadal non-endometrial YST are similar to those of YST arising from the ovary. Patients with advanced recurrent ovary YST can benefit from chemotherapy combined targeted antiangiogenic drugs and immune therapy.
Acknowledgments
Funding: This study received support from the Wuxi Science and Technology Development Foundation (N20202020).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-663
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-663). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Approval was obtained from the ethics committee of Wuxi Maternal and Child Health Care Hospital Ethics Committee (document number: 2021-01-0202-04). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miller KD, Michael H, Jacobson L, et al. Primary yolk sac tumor of the rectum. Cancer Invest 2000;18:597-601. [Crossref] [PubMed]
- Sicari MC, Fyfe B, Parness I, et al. Intrapericardial yolk sac tumor associated with acute myocarditis. Arch Pathol Lab Med 1999;123:241-3. [Crossref] [PubMed]
- Wang C, Li G, Xi L, et al. Primary yolk sac tumor of the endometrium. Int J Gynaecol Obstet 2011;114:291-3. [Crossref] [PubMed]
- Clement PB, Young RH, Scully RE. Extraovarian pelvic yolk sac tumors. Cancer 1988;62:620-6. [Crossref] [PubMed]
- Zaczek T. Mesonephric carcinoma of the cervix uteri in an 11-month-old girl treated by hysterectomy. Am J Obstet Gynecol 1963;85:176-8. [Crossref] [PubMed]
- Saltzman AF, Gills JRR, LeBlanc DM, et al. Multimodal management of a pediatric cervical yolk sac tumor. Urology 2015;85:1186-9. [Crossref] [PubMed]
- Chen LZ. editor. Diagnostic pathology of obstetrics and gynecology. Beijing: People's Military Medical Publishing House, 2002.
- Shokeir MO, Noel SM, Clement PB. Malignant müllerian mixed tumor of the uterus with a prominent alpha-fetoprotein-producing component of yolk sac tumor. Mod Pathol 1996;9:647-51. [PubMed]
- Pasternack T, Shaco-Levy R, Wiznitzer A, et al. Extraovarian pelvic yolk sac tumor: case report and review of published work. J Obstet Gynaecol Res 2008;34:739-44. [Crossref] [PubMed]
- Nogales FF, Preda O, Nicolae A. Yolk sac tumours revisited. A review of their many faces and names. Histopathology 2012;60:1023-33. [Crossref] [PubMed]
- Damato S, Haldar K, McCluggage WG. Primary endometrial yolk sac tumor with endodermal-intestinal differentiation masquerading as metastatic colorectal adenocarcinoma. Int J Gynecol Pathol 2016;35:316-20. [Crossref] [PubMed]
- Qzler A, Dogan S, Mamedbeyli G, et al. Primary yolk sac tumor of endometrium: report of two cases and review of literature. J Exp Ther Oncol 2015;11:5-9. [PubMed]
- Yang ZJ, Liu ZC, Wei RJ, et al. An analysis of prognostic factors in patients with ovarian malignant germ cell tumors who are treated with fertility-preserving surgery. Gynecol Obstet Invest 2016;81:1-9. [Crossref] [PubMed]
- Simpson S, Simoni M, Hui P, et al. Extragonadal yolk sac tumor limited to the myometrium: report of a case with potential fertility preservation and molecular analysis suggesting germ cell origin. Int J Gynecol Pathol 2020;39:247-53. [Crossref] [PubMed]
(English Language Editor: J. Jones)


