
Prepectoral breast reconstruction: an ideal approach to bilateral risk-reducing mastectomy
Introduction
Prophylactic mastectomy has become a hot topic due to increasingly offered genetic counselling and further understanding of molecular specifics of breast cancer. Bilateral risk-reducing mastectomy (BRRM) aims at minimising the chances of developing breast cancer in women at high risk (1,2).
Following the recommendations of the National Comprehensive Cancer Network (NCCN), this procedure is provided to patients with demonstrated breast cancer-related gene mutations, such as BRCA1 and BRCA2, as well as to patients with a strong family history of breast cancer and to patients with a biopsy-confirmed high-risk histology (3).
In this scenario, oncoplastic surgery plays a huge role in breast reconstruction having the responsibility to combine preventive, aesthetical and functional desires of women who have not yet developed the disease.
The surgical approach to these patients has changed considerably in the last decade. An increasing attention has been paid to the psychological comfort provided by nipple-sparing mastectomy with immediate breast reconstruction (4-9). Furthermore, changing the plane of the reconstruction from subpectoral to prepectoral along with the possibility to completely cover the breast implant with a sheet of acellular dermal matrix (ADM) represent some of the most recent advancements in the field of less invasive and less disabling surgery.
Hence, the prepectoral breast reconstruction (PPBR) with ADM provides a full-muscle-sparing immediate reconstruction which reduces pain, eliminates animation deformity and avoids direct contact of the implant with the subcutaneous layer, reducing capsular contracture and resulting in high patient satisfaction (10,11).
This study aims to present our single-centre experience in performing prepectoral breast reconstruction with ADM following bilateral prophylactic mastectomy on patients defined at high risk of developing breast cancer. Patients’ satisfaction, aesthetic results, functional impairment and short and long-term postoperative complications were evaluated and are here reported.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-339).
Methods
A prospective data collection was carried out from January 2017 to January 2021, in all consecutive patients who received bilateral prophylactic mastectomy and underwent prepectoral implant-based breast reconstruction with Braxon® acellular dermal matrix (GmbH, Germany, under the license of Decomed S.r.l., Italy).
All patients that fit the following criteria were considered candidates for the proposed procedure: (I) Patients undergoing bilateral prophylactic nipple-sparing mastectomy for: (i) demonstrated breast cancer-related gene mutation, such as BRCA1, BRCA2, PTEN, TP53, STK11, CDH1 or ATM; (ii) a family history of breast cancer in multiple first-degree relatives and/or family members with breast and/or ovarian cancer (family cancer syndromes); (iii) a biopsy-confirmed high-risk histology (atypical ductal hyperplasia; atypical lobular hyperplasia; lobular carcinoma in situ). (II) Patients undergoing immediate reconstruction who meet criteria for prepectoral implantation with complete ADM coverage (12,13).
Exclusion criteria include: (I) patients found to have occult cancer at the time of surgery; (II) patients who underwent contralateral risk-reducing mastectomy; (III) patients with breast cancer or a history of breast cancer; (IV) patients with history of prior mantle irradiation.
Patients’ age, body mass index (BMI), time of procedure, weight of breast tissue removed, implant size, comorbidities, related ASA physical status and smoking habits were recorded (Table 1).
Table 1
| Characteristics | Values |
|---|---|
| Total No. of patients | 23 |
| Total No. of breasts | 46 |
| Average age, years [range] | |
| Group A | 42.1 [38–54] |
| Group B | 49.1 [40–63] |
| Average BMI (kg/m2) [range] | |
| Group A | 23.05 [19.47–27] |
| Group B | 31.5 [28.7–43.1] |
| Diabetes | |
| Yes | 2 |
| No | 21 |
| Smoking habit | |
| Yes | 4 |
| No | 19 |
| ASA | |
| 1 | |
| Group A | 9 |
| Group B | 1 |
| 2 | |
| Group A | 8 |
| Group B | 5 |
| 3 | 0 |
| Indication for surgery | |
| BRCA1 | 6 |
| BRCA2 | 8 |
| PTEN | 1 |
| P53 | 1 |
| Family history | 3 |
| Biopsy-confirmed high-risk | 4 |
| Average nipple-to-sternal notch distance (SD) [range] | |
| Group A | 24.3 (3.2) [19–29.4] |
| Group B | 29.8 (3.6) [26–35] |
| Mean hospital stay (SD) [range] | 2.5 (0.8) [2–4] |
| Average follow-up, months (SD) [range] | 18.4 (3.2) [12–31] |
| Breast shape | |
| Large ptotic breasts | 6 |
| Normally shaped breasts | 17 |
| Duration of complete procedure (min) | |
| Group A | 215.5 [165–315] |
| Group B | 250 [195–340] |
| Mastectomy specimen weight (g) | |
| Group A | |
| Right breast [range] | 250.2 [110–450] |
| Left breast [range] | 238.5 [100–463] |
| Group B | |
| Right breast [range] | 679.2 [512–850] |
| Left breast [range] | 688.5 [500–943] |
| Average permanent implant volume (cc) [range] | |
| Group A | 295.5 (50.0) [250–350] |
| Group B | 415.5 (55.5) [375–500] |
| Breast implant profile (No) | |
| Round | 14 |
| Anatomical | 32 |
| Incision mastectomy pattern (No of breasts) | |
| IMF [average length, cm (range)] | 34 [5.3 (4.5–6)] |
| Wise pattern | 12 |
| NAC graft No. of breasts | 2 |
| NAC flap No. of breasts | 10 |
BMI, body mass index; ASA, American Society of Anesthesiologists; SD, standard deviation; NAC, nipple-areola-complex.
Patient were divided into two groups: (I) Group A: patients with small and medium size breasts; (II) Group B: patients with large and ptotic breasts.
All patients were strictly followed with periodic control visits at 1, 3, 6, 12, and 18 months. The occurrence of postoperative complications defined as hematoma, seroma, wound dehiscence, red breast syndrome, rippling, implant loss, partial and total nipple necrosis, were noted during each check-up. The occurrence of capsular contracture was assessed during each visit using the Baker classification (14) (Table 2).
Table 2
| Complication | No of breast | % |
|---|---|---|
| Hematoma | 1 | 2.17 |
| Seroma | 1 | 2.17 |
| Wound dehiscence | 2 | 4.35 |
| NAC necrosis | ||
| Partial | 0 | 0 |
| Complete | 0 | 0 |
| Rippling | 0 | 0 |
| Red breast syndrome | 0 | 0 |
| Implant loss | 0 | 0 |
| Capsular contracture | 0 | 0 |
NAC, nipple-areola-complex.
The preoperative and the postoperative BREAST-Q reconstructive modules were administered to evaluate Health-related quality of life (HRQOL) of patients undergoing this procedure. The preoperative questionnaire was provided to patients 1 month before surgery whereas the postoperative one was administered 1 year after the completion of the reconstruction, during the programmed clinic visit (15) (Table 3).
Table 3
| Domain | 1 month preoperatively | 1 year postoperatively | P value | |||
|---|---|---|---|---|---|---|
| N | Mean SD | N | Mean SD | |||
| Satisfaction with breasts | 23 | 70.218.6 | 23 | 77.215.6 | <0.05 | |
| Psychosocial well-being | 23 | 75.418.7 | 23 | 88.319.7 | <0.05 | |
| Physical well-being | 23 | 69.213.4 | 23 | 73.212.8 | <0.05 | |
| Sexual well-being | 23 | 59.615.2 | 23 | 69.818.5 | <0.05 | |
Women who have undergone BRRM with direct-to-implant (DTI) ADM reconstruction were exhaustively informed of all possible risks and benefits of the procedure. All the other available reconstructive techniques, such as traditional subpectoral expander-implant technique or autologous reconstruction were illustrated to them and the best option was chosen according to the patient’s clinical needs.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Policlinico Hospital of Bari (No. 6422) and informed consent was taken from all the patients
Operative technique
All the procedures were performed jointly by oncological and reconstructive surgeons.
The patients in Group A underwent a standard bilateral nipple-sparing mastectomy (NSM) through a horizontal incision at the inframammary fold (IMF) (Figure 1). The following reconstruction was a one-stage PPBR. A Wise pattern nipple-sparing skin reducing mastectomy with de-epithelialisation of an inferior adipo-dermal flap was the chosen technique for Group B patients (Figure 2). PPBR was then performed covering the implant with the ADM and the inferior dermal sling, as previously described by the authors (16,17).
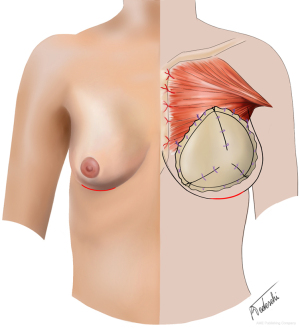
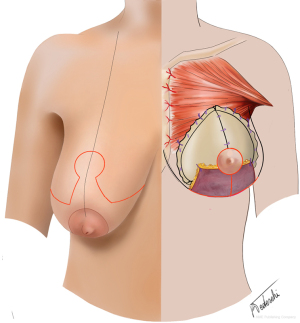
The nipple-areola complex was preserved, and it was either on a deepithelialized superiorly-based pedicle or harvested as a full-thickness skin graft and grafted to the new position, depending on the preoperative sternum-nipple distance and the intraoperative ICG findings.
In both groups an intraoperative frozen examination of the retroareolar tissue was performed before completing the procedure. Further, intraoperative evaluation of flaps perfusion was assessed using indocyanine green dye laser-induced fluorescence imaging as an adjunctive tool (16). If perfusion of the mastectomy skin flaps was inadequate, an under-filled expander was inserted in a submuscular pocket and patients were excluded from this study. The absence of cancer in the bilateral mastectomy specimens was confirmed by routine histological analysis.
Perioperative antibiotics (teicoplanin 800 mg) were administered 30 minutes before the surgical incision and two doses (teicoplanin 400 mg) in the 24 h after surgery.
One suction drain (Jackson Pratt® 7 4/4) was inserted into the prepectoral pocket and kept until drainage was less than 20–25 cc/daily for 2 days.
After surgery, all patients were instructed to wear a good-fitting sports bra for at least 4 weeks during day and nighttime and for the following 10 weeks at least during daytime.
Statistical analysis
Multiple linear regression analysis was used to assess associations of QOL with patients’ demographic and clinical characteristics. Data were analysed using the Statistical Package for Social Sciences (SPSS) software for Windows, version 23.0 (IBM SPSS, IBM Corp., Armonk, NY, USA).
Results
Twenty-five patients were candidate to bilateral prophylactic nipple-sparing mastectomy. Twenty-three patients met the inclusion criteria of the study. Two patients were excluded due to an unreasonable subcutaneous layer over the breast tissue.
Group A accounted for 17 patients; the remaining 6 women were included in Group B. The mean follow-up was 18.4 months (range, 12 to 31 months).
In group A the median age was 42.1 (range, 38–54) and in group B was 49.1 (range, 40–63). The median body mass index was 23.05 kg/m2 (range, 19.47–27 kg/m2) in Group A, and 31.5 kg/m2 (range, 28.7–43.1 kg/m2) in Group B. The most common indication for risk-reducing bilateral NSM for patients in this series was mutation in a breast cancer-associated gene (16 patients, 69.5%): BRCA1 (26.1%), BRCA2 (34.8%), PTEN (4.3%), TP53 (4.3%). Other indications included a strong family history of breast cancer (13%), lobular carcinoma in situ/atypia (17.3%). Ten patients were ASA I (healthy, no smoking); 13 patients were ASA II (smoker for less than 10 years, and/ or obese, and/ or well controlled diabetic). Both anatomical (n=32) and round (n=14) shaped silicone implants were employed. The average implant size was 295.5 cc (range, 250 to 350 cc) in Group A and 415.5 cc (range, 375 to 500 cc) in Group B. A total number of 46 ADMs has been used. The operative time for mastectomy and DTI reconstruction was on average 215.5 minutes (range, 315 to 165 minutes) in Group A and 250 minutes (range, 340 to 195 minutes) in Group B. Breast weight after mastectomy ranged in group A from 100 to 463 gr (mean 238.5 gr), in Group B from 500 to 943 gr (mean 688.5 gr).
When a wise pattern skin reducing mastectomy was performed (Group B), NAC was transposed based on a deepithelialized superiorly based pedicle in 5 patients (notch-NAC distance range, 25–29 cm), while it was harvested as a full-thickness skin graft in one case (nipple-areola distance: 35 cm bilaterally).
The patients were discharged 2 days after surgery (mean hospital stay 2.5 days, range, 2–4).
Minor complications occurred in four breasts: two small wound dehiscence, one seroma and one hematoma. All cases of minor complications were managed conservatively and did not determine an unplanned return to the operating room. No NAC necrosis was observed. No implant loss was observed. No significant capsular contracture (grade III or IV) was detected at 18 months follow-up.
BREAST-Q questionnaire results for the self-reported measures of health-related quality of life are represented in Table 3. Patients scored high levels of satisfaction with outcome. Overall satisfaction with breasts, psychosocial, physical and sexual well-being all significantly increased after surgery (P<0.05) (Figure 3).
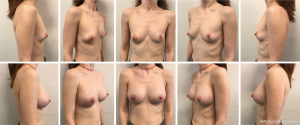
Three patients belonging to Group A underwent an additional lipofilling procedure 1 year after surgery in order to improve upper pole fullness and reduce rippling sign.
No patients undergoing bilateral risk-reducing NSM in this series developed breast cancer during follow-up.
Discussion
Breast cancer represents the most common tumour among women and affects nearly every woman either personally or through a family member or a friend (18).
Nowadays, there are several procedures that allow clinicians to perform detailed genetic screenings, understand risk factors and identify information about the probability of developing cancer. Patients with BRCA1 or BRCA2 mutations have a higher chance of developing breast cancer, with a lifetime risk of 49–82% (19-21). BRCA mutations are the most common indication for BRRM, but patients with PTEN, TP53 and ATM mutations should also be considered as they have a significantly increased risk of breast cancer up to 85%, 90% and 60% respectively (22,23).
Moreover, the psychological effect of dealing with breast cancer should be kept in mind. A woman who has seen a family member or friend struggle with breast cancer faces a looming cloud of uncertainty about how the disease may or may not affect her.
Bilateral risk-reduction surgery has been shown to reduce the incidence of breast cancer by up to 95% and has become increasingly common concomitantly with the improved ability to objectively assess the cancer risk of individual patients (24-27).
Graves and Metcalfe investigated the psychological impact of a positive BRCA test on women and indicated a decline in cancer-related distress within 2 years in women who chose to have BRRM instead of just surveillance (28,29). Interventional surgery confers a psychological benefit as well as a real reduction of the cancer occurrence and it should be offered to women who have been objectively determined at high risk.
Women undergoing a prophylactic procedure must be considered highly demanding patients and represent a real challenge for oncoplastic surgeons given the need to integrate the preventive desire with a good aesthetic result and no functional damage. Therefore, the reconstructive technique should be minimally invasive, ideally performed in a single stage with early discharge and rapid recovery.
Gahm et al. in 2010 and Isaksson et al. in 2019 reported their experience on patients who had undergone BRRM with concomitant breast reconstruction. In these studies, the authors performed breast reconstructions using a permanent tissue expander or a permanent implant in a sub-pectoral plane (30,31). This reconstructive modality has several disadvantages that could not suit demanding patients, including the multiple visits for expansion, post-operative pain and shoulder impairment (32-35).
Lim et al. proposed a new approach for BRRM with a two-stage procedure, with the initial placement of the implant in a dual plane fashion and performing a bilateral mastectomy only after the creation of a periprosthetic capsule. This procedure presented some disadvantages, such as patient discomfort associated with a staged procedure and poor oncological safety, because implant placement before BRRM can lead to a potential delay in breast cancer treatment (36).
With the advent of ADMs, DTI prepectoral reconstruction has re-emerged as an excellent technique for post-mastectomy reconstruction (37). The main drawback of the procedure is the risk of soft tissue deficiency over the implant such as rippling and implant visibility or palpability, especially in the upper pole. Still the advantages are countless. Indeed, the benefits of this technique have been extensively discussed in the literature and include a reduction of postoperative pain and discomfort, no need for postoperative expansion, definitive disappearance of animation deformity, reduction of capsular contracture rates, better aesthetic results without any negative impact on the upper limbs function (38-49).
All these considerations explain why we thought that single stage prepectoral breast reconstruction could ideally fit our study population.
Patients with large ptotic breasts deserve a specific mention. Considering the advantages of one-stage prepectoral breast reconstruction, we decided to offer such surgical intervention also to women with large and ptotic breasts who decided to undergo BRRM. In this group of patients, we have combined nipple-sparing mastectomy with prepectoral implantation and full ADM coverage with inferior adipo-dermal flap. The de-epithelialized dermal flap presumably obviates wound complications by protecting the underlying implant-ADM and improving the reliability of the reconstruction (16). Patients with longer hospitalization belong to this group, as they may require mastectomy flaps’ and NAC monitoring.
In our cohort, all patients scored high in the physical well-being domain of the Breast-Q at one-year post-op (73.2±12.8, P<0.05). Indeed, we found significant higher rates in the BREAST-Q concerning satisfaction with breasts (77.2±15.6, P<0.05), psychosocial (88.3±19.7, P<0.05) and sexual well-being (69.8±18.5, P<0.05) domains as well, confirming patients’ satisfaction with the surgical result (Table 3).
Moreover, during the study period no major complications were encountered. This data is coherent with the findings already published in literature, as described by Azouz et al. in 2017. From a comparison of direct-to-implant with two-stage tissue expander–implant breast reconstructions, they observed that multiple surgeries are associated with an increased complication risk, such as high infection rates (13.6% in DTI vs. 30.3% in two-stage BR) or implant loss (1.7% in DTI vs. 6.3% in two-stage BR) (50).
No significant capsular contracture (grade III or IV) was detected in a mean follow-up of 18 months. Clinical evidence suggest that visibility of the superior pole of the implant and rippling could be problems associated with the prepectoral technique, but none of these complications have been observed.
The mastectomy incision was also precisely chosen. Surgical access through the IMF was performed on the 17 patients belonging to group A, with an incision of 5.3 cm average length (range, 4.5–6 cm). No complications related to this access was encountered. Instead, a Wise pattern incision with inverted T sutures was performed in the 6 patients belonging to group B. The horizontal incision at the level of the IMF or the inverted T suture recall the surgical accesses used for breast augmentation/mastopexy in aesthetic surgery and positively influence the acceptance of the patient's body image.
An experienced breast surgeon who preserves the subcutaneous layer of the mastectomy flaps and the perforators that vascularize them is the key to success of this surgery. To a degree, the thickness of the subcutaneous layer can be evaluated preoperatively using digital mammography or magnetic resonance imaging. Moreover, most authors clinically assess flap vascularity based on colour, absence of dermal exposure and flap damage from diathermy. If devices to assess skin flap vascularity are available, the authors would advise to use it, particularly in situations when there is uncertainty about flap viability.
Some doctors avoid the use of ADMs because of their cost, but as Glasberg et al. theorized, although there is an increase in the cost of reconstructive materials, prepectoral reconstruction ultimately proves to be cost-effective since there are no further surgical steps, the need of physical therapy decreases, and the reduced drug use allows a faster return to work, and a shorter length of hospital stay (51). A pilot cost analysis using the same ADM of the presented paper has shown that pre-pectoral breast reconstruction has a potential cost benefit compared with sub-pectoral one, especially for bilateral reconstructions (52).
Our experience represents the first case series in the literature of patients undergoing prepectoral immediate breast reconstruction with ADM following bilateral prophylactic mastectomy.
A larger sample size with a longer follow-up is warranted to confirm our results.
Conclusions
Prepectoral breast reconstruction could represent the ideal reconstruction option after BRRM as it represents a full-muscle-sparing technique with no sacrifice of a woman’s pectoralis major muscle, or any other key element of her body. Moreover, it provides good aesthetic results in terms of shape, volume and symmetry with low postoperative complications.
Accurate patient selection is critical to reduce the risk of postoperative complications.
In conclusion, we suggest offering single-stage ADM prepectoral breast reconstruction to all women undergoing BRRM that fulfil the inclusion criteria.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-339
Data Sharing Statement: Available at https://dx.doi.org/10.21037/gs-21-339
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-339). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Policlinico Hospital of Bari (No. 6422) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2004;22:1055-62. [Crossref] [PubMed]
- NCCN clinical practice guidelines in oncology, breast cancer risk reduction. 2020. Available online: https://www.nccn.org/professionals/physician_gls/ pdf/breast_risk.pdf
- Geiger AM, Nekhlyudov L, Herrinton LJ, et al. Quality of life after bilateral prophylactic mastectomy. Ann Surg Oncol 2007;14:686-94. [Crossref] [PubMed]
- Brandberg Y, Sandelin K, Erikson S, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol 2008;26:3943-9. [Crossref] [PubMed]
- Panchal H, Matros E. Current Trends in Postmastectomy Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Djohan R, Gage E, Gatherwright J, et al. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg 2010;125:818-29. [Crossref] [PubMed]
- Wei CH, Scott AM, Price AN, et al. Psychosocial and Sexual Well-Being Following Nipple-Sparing Mastectomy and Reconstruction. Breast J 2016;22:10-7. [Crossref] [PubMed]
- Maruccia M, Mazzocchi M, Dessy LA, et al. One-stage breast reconstruction techniques in elderly patients to preserve quality of life. Eur Rev Med Pharmacol Sci 2016;20:5058-66. [PubMed]
- Masià J. iBAG Working Group. The largest multicentre data collection on prepectoral breast reconstruction: The iBAG study. J Surg Oncol 2020;122:848-60. [PubMed]
- Maruccia M, Di Taranto G, Onesti MG. One-stage muscle-sparing breast reconstruction in elderly patients: A new tool for retaining excellent quality of life. Breast J 2018;24:180-3. [Crossref] [PubMed]
- Martin L, O'Donoghue JM, Horgan K, et al. Acellular dermal matrix (ADM) assisted breast reconstruction procedures: joint guidelines from the Association of Breast Surgery and the British Association of Plastic, Reconstructive and Aesthetic Surgeons. Eur J Surg Oncol 2013;39:425-9. [Crossref] [PubMed]
- Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Spear SL, Baker JL Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg 1995;96:1119-23; discussion 1124. [Crossref] [PubMed]
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 2009;124:345-53. [Crossref] [PubMed]
- Maruccia M, Elia R, Gurrado A, et al. Skin-Reducing Mastectomy and Pre-pectoral Breast Reconstruction in Large Ptotic Breasts. Aesthetic Plast Surg 2020;44:664-72. [Crossref] [PubMed]
- Bucaria V, Elia R, Maruccia M, et al. Why Choose the Septum-Supero-Medial (SSM)-Based Mammaplasty in Patients with Severe Breast Ptosis: An Anatomical Point of View. Aesthetic Plast Surg 2018;42:1439-46. [Crossref] [PubMed]
- Coughlin SS. Epidemiology of Breast Cancer in Women. Adv Exp Med Biol 2019;1152:9-29. [Crossref] [PubMed]
- Evans DG, Shenton A, Woodward E, et al. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer 2008;8:155. [Crossref] [PubMed]
- Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003;72:1117-30. [Crossref] [PubMed]
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol 2015;22:370-6. [Crossref] [PubMed]
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 2015;372:2243-57. [Crossref] [PubMed]
- Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res 2012;18:400-7. [Crossref] [PubMed]
- Eisemann BS, Spiegel AJ. Risk-Reducing Mastectomy and Breast Reconstruction: Indications and Evidence for Current Management Strategies. Clin Plast Surg 2018;45:129-36. [Crossref] [PubMed]
- Baildam AD. Current knowledge of risk reducing mastectomy: Indications, techniques, results, benefits, harms. Breast 2019;46:48-51. [Crossref] [PubMed]
- Manning AT, Wood C, Eaton A, et al. Nipple-sparing mastectomy in patients with BRCA1/2 mutations and variants of uncertain significance. Br J Surg 2015;102:1354-9. [Crossref] [PubMed]
- Jakub JW, Peled AW, Gray RJ, et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg 2018;153:123-9. [Crossref] [PubMed]
- Metcalfe KA, Mian N, Enmore M, et al. Long-term follow-up of Jewish women with a BRCA1 and BRCA2 mutation who underwent population genetic screening. Breast Cancer Res Treat 2012;133:735-40. [Crossref] [PubMed]
- Graves KD, Vegella P, Poggi EA, et al. Long-term psychosocial outcomes of BRCA1/BRCA2 testing: differences across affected status and risk-reducing surgery choice. Cancer Epidemiol Biomarkers Prev 2012;21:445-55. [Crossref] [PubMed]
- Gahm J, Jurell G, Edsander-Nord A, et al. Patient satisfaction with aesthetic outcome after bilateral prophylactic mastectomy and immediate reconstruction with implants. J Plast Reconstr Aesthet Surg 2010;63:332-8. [Crossref] [PubMed]
- Isaksson K, Arver B, Bottai M, et al. Bilateral Risk-Reducing Mastectomies with Implant-Based Reconstructions Followed Long Term: A Consecutive Series of 185 Patients. World J Surg 2019;43:2262-70. [Crossref] [PubMed]
- Ter Louw RP, Nahabedian MY. Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:51S-59S. [Crossref] [PubMed]
- Cattelani L, Polotto S, Arcuri MF, et al. One-Step Prepectoral Breast Reconstruction With Dermal Matrix-Covered Implant Compared to Submuscular Implantation: Functional and Cost Evaluation. Clin Breast Cancer 2018;18:e703-11. [Crossref] [PubMed]
- Giudice G, Maruccia M, Nacchiero E, et al. Dual plane breast implant reconstruction in large sized breasts: How to maximise the result following first stage total submuscular expansion. JPRAS Open 2018;15:74-80. [Crossref] [PubMed]
- Salibian AA, Frey JD, Karp NS. Strategies and considerations in selecting between subpectoral and prepectoral breast reconstruction. Gland Surg 2019;8:11-8. [Crossref] [PubMed]
- Lim GH, Baildam AD. Novel Approach for Risk-Reducing Mastectomy: First-Stage Implant Placement and Subsequent Second-Stage Mastectomy. Plast Reconstr Surg 2018;142:607-10. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: a new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [Crossref] [PubMed]
- Jones G, Antony AK. Single stage, direct to implant pre-pectoral breast reconstruction. Gland Surg 2019;8:53-60. [Crossref] [PubMed]
- Hammond DC, Schmitt WP, O'Connor EA. Treatment of breast animation deformity in implant-based reconstruction with pocket change to the subcutaneous position. Plast Reconstr Surg 2015;135:1540-4. [Crossref] [PubMed]
- Kobraei EM, Cauley R, Gadd M, et al. Avoiding Breast Animation Deformity with Pectoralis-Sparing Subcutaneous Direct-to-Implant Breast Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e708 [Crossref] [PubMed]
- Salzberg CA, Ashikari AY, Berry C, et al. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg 2016;138:329-37. [Crossref] [PubMed]
- Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral Revision Breast Reconstruction for Treatment of Implant-Associated Animation Deformity: A Review of 102 Reconstructions. Aesthet Surg J 2018;38:519-26. [Crossref] [PubMed]
- Antony AK, Robinson EC. An Algorithmic Approach to Prepectoral Direct-to-Implant Breast Reconstruction: Version 2.0. Plast Reconstr Surg 2019;143:1311-9. [Crossref] [PubMed]
- Baker BG, Irri R, MacCallum V, et al. A Prospective Comparison of Short-Term Outcomes of Subpectoral and Prepectoral Strattice-Based Immediate Breast Reconstruction. Plast Reconstr Surg 2018;141:1077-84. [Crossref] [PubMed]
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [Crossref] [PubMed]
- Highton L, Johnson R, Kirwan C, et al. Prepectoral Implant-Based Breast Reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1488 [Crossref] [PubMed]
- Vidya R, Green M. Minimal Pain with Prepectoral Implant-Based Breast Reconstruction. Plast Reconstr Surg 2019;143:236e. [Crossref] [PubMed]
- Onesti MG, Maruccia M, Di Taranto G, et al. Clinical, histological, and ultrasound follow-up of breast reconstruction with one-stage muscle-sparing "wrap" technique: A single-center experience. J Plast Reconstr Aesthet Surg 2017;70:1527-36. [Crossref] [PubMed]
- Marcasciano M, Kaciulyte J, Mori FLR, et al. Breast surgeons updating on the thresholds of COVID-19 era: results of a multicenter collaborative study evaluating the role of online videos and multimedia sources on breast surgeons education and training. Eur Rev Med Pharmacol Sci 2020;24:7845-54. [PubMed]
- Azouz V, Lopez S, Wagner DS. Surgeon-Controlled Comparison of Direct-to-Implant and 2-Stage Tissue Expander-Implant Immediate Breast Reconstruction Outcomes. Ann Plast Surg 2018;80:212-6. [Crossref] [PubMed]
- Glasberg SB. The Economics of Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:49S-52S. [Crossref] [PubMed]
- Garreffa E, Agrawal A. Cost-effectiveness of pre-pectoral implant-based breast reconstruction: A pilot comparative analysis. J Plast Reconstr Aesthet Surg 2019;72:1700-38. [Crossref] [PubMed]


