
Metastatic liver cancer with hormone secretion: a case report
Introduction
Adrenocortical carcinoma (ACC) is a rare and challenging cancer with an incidence of 0.72 per million cases per year (1) and with a poor prognosis (2). ACC affects females more frequently than males (1.5:1) (3). The patients usually suffer from primary or metastatic tumors, and 40–60% of the tumors have hormone secretion function to cause Cushing’s syndrome (CS) and hypercortisolism (4). Unfortunately, metastatic and recurrent of ACC exhibit poor 5-survival rates of less than 15% (2), the optimal management and clinical outcomes of ACC remain ill-defined. Due to the failure of effective treatment, surgery remains the main treatment for ACC, which includes distant resectable metastases and ACC recurrent (2).
It has not been well studied on the ACC due to its rarity. This case report is expected to share the experience of the rare case through a patient with CS caused by adrenocorticotropic hormone (ACTH) secretion from an ACC metastatic to the liver. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/gs-21-296).
Case presentation
A 34-year-old Chinese woman was admitted to our hospital with a liver tumor for 2 months. Two years ago, she was diagnosed with left-sided ACC at another hospital and then underwent left adrenalectomy for ACC. In the past 2 years, she has not had any post-operative follow-up.
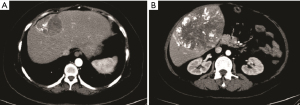
She had several months of pain in the liver area and abdominal distention. Physical examination revealed a moon-like face and central obesity; the blood pressure was 160/110 mmHg in supine position; the weight was 80 kg with body mass index (BMI) of 31.2 kg/m2; laboratory evaluation revealed elevated blood glucose, triglycerides, cortisol, and aldosterone (Table 1); contrast-enhanced computed tomography (CT) revealed two round lesions 65×51 and 135×105 mm in size (Figure 1) and no tumor was found in the bilateral adrenal areas on CT.
Table 1
| Variables | Preoperative | Postoperative | Reference range |
|---|---|---|---|
| Blood pressure (mmHg) | 160/110 | 130/80 | 90–139/60–89 |
| AFP (ng/mL) | 4.2 | – | <9.0 |
| Glucose (mmol/L) | 15.06 | 8.99 | 3.9–6.1 |
| Triglycerides (mmol/L) | 5.15 | – | 0.56–1.71 |
| Cortisol (5–7 AM) (ng/mL) | 587.04 | 134.22 | 66–268 |
| Cortisol (3–5 PM) (ng/mL) | 520.23 | – | 22–154 |
| Aldosterone (Standing) (pg/mL) | 162.48 | – | 63–239.6 |
| Aldosterone (recumbant) (pg/mL) | 151.37 | 74.07 | 48.5–123.5 |
| ACTH, adrenocorticotropic hormone (pg/mL) | – | 56.80 | 0.10–46 |
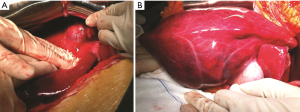
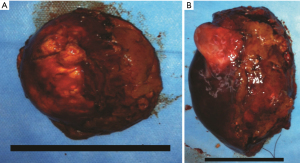
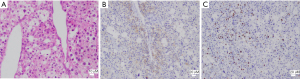
Tumors were completely removed (Figures 2,3). The histopathological examination was consistent with liver metastasis of the ACC (Figure 4). Two days after the operation, she had agitation, delirium, irritability, and other psychological symptoms probably due to the abrupt drop of cortisol (Table 1). Her symptoms gradually improved after the administration of 20 mg of dexamethasone. Subsequently, she was given daily hydrocortisone, and the dose was slowly tapered over the next week. Aldosterone concentrations gradually dropped back to the normal range with normal blood pressure (Table 1). She was discharged from the hospital 10 days after surgery without any severe complications. Unfortunately, six months after the hepatectomy, she eventually died due to progressive deterioration and the refusal of further treatment.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
ACC is a rare and challenging cancer with a heterogeneous presentation and the optimal management and clinical outcomes of ACC remain ill-defined due to the rarity of the disease (2,5,6). According to the European Network for the Study of Adrenal Tumors (ENSAT) criteria, the case could be classified into ENSAT stage IV (7).
For patients with metastatic ACC (ENSAT stage IV), the 5-year overall survival rate is less than 15% (2), with a median survival of 6 to 20 months (8,9). There is still no optimal management for metastatic ACC, and data on the effectiveness of radiotherapy and chemotherapy for ACC remain limited (10,11). Thus, the surgical approach remains the best-recommended treatment modality as long as the metastases of ACC are resectable completely (2). Some studies have suggested that surgical resection of the metastasis or recurrent for ACC is associated with prolonged survival in selected patients (5,12). However, a complete cure is unlikely to be achieved after metastasectomy. Another study claimed that all 28 patients who underwent hepatectomy for liver metastasis from ACC developed recurrent disease (13). In this case, 6 months after hepatic metastasectomy, the patient died due to progressive deterioration and refusal of further treatment. If the patient were treated promptly including surgical treatment for recurrent disease (if applicable), she probably had survived longer than 6 months. Studies have also shown that repeated resection for ACC could provide prolonged survival (13).
It has been reported that 40–60% of ACC are functioning tumors (4) that secrete cortisol (50%) most commonly followed by androgens, while aldosterone secretion is quite rare (<2%) (8). During the perioperative period, the concentration of hormones secreted by functional tumors fluctuates. Other studies reported that plasma ACTH levels began to decline significantly within 10–15 min of ectopic tumor resection (14). In this case, the concentrations of cortisol and aldosterone were elevated preoperatively. After liver metastasectomy, the patient suffered from transient psychological symptoms due to a sudden drop in hormone levels, and needed hormone replacement therapy. Following the hormone replacement therapy, her symptoms gradually improved and the dose was slowly tapered over the next week. Assessment of quantitative and/or qualitative changes in hormone secretion plays an important role for the patients. Increased cortisol secretion by functioning tumors is described as a poor prognostic factor for ACC (15). Steroid profiles can be used as tumor markers to detect metastatic disease (16). In this case, if she had been provided with prompt serial hormonal assessments after the primary adrenalectomy for ACC, it would have been possible to detect the metastatic tumor in the liver as soon as possible and improve the patient’s prognosis.
A German retrospective study showed single metastasis was a predictor of significantly prolonged survival compared with multiple metastases (2). The poor prognosis of this patient may be associated with multiple metastases in the liver.
As mentioned above,the cancer stage (ENSAT stage IV), functional tumor and multiple metastases are poor prognostic factors in this patient. However, other studies have reported that left ACC and disease-free interval (DFI) greater than 1 year are associated with prolonged survival. DFI is defined as the time to the first recurrence at any site after the initial surgery (4). Although the case was a left-sided primary ACC with DFI of about 2 years, the prognosis was relatively poor. This may be associated with the absence of serial follow-up after primary adrenalectomy and liver metastasectomy, which includes prompt serial hormonal assessments,abdominal CT, magnetic resonance imaging, and adequate thoracic CT imaging (5). As the optimal treatment and clinical outcomes of ACC remain ill-defined, prompt serial follow-up after the operation is probably crucial for a better prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/gs-21-296
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/gs-21-296). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Owen DH, Patel S, Wei L, Phay JE, Shirley LA, Kirschner LS, Schmidt C, Abdel-Misih S, Brock P, Shah MH, Konda B. Metastatic Adrenocortical Carcinoma: a Single Institutional Experience. Horm Cancer 2019;10:161-7. [Crossref] [PubMed]
- Baur J, Büntemeyer TO, Megerle F, et al. Outcome after resection of Adrenocortical Carcinoma liver metastases: a retrospective study. BMC Cancer 2017;17:522. [Crossref] [PubMed]
- Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer 2008;113:3130-6. [Crossref] [PubMed]
- Datrice NM, Langan RC, Ripley RT, et al. Operative management for recurrent and metastatic adrenocortical carcinoma. J Surg Oncol 2012;105:709-13. [Crossref] [PubMed]
- Erdogan I, Deutschbein T, Jurowich C, et al. The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab 2013;98:181-91. [Crossref] [PubMed]
- Lo WM, Kariya CM, Hernandez JM. Operative Management of Recurrent and Metastatic Adrenocortical Carcinoma: A Systematic Review. Am Surg 2019;85:23-8. [Crossref] [PubMed]
- Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 2009;115:243-50. [Crossref] [PubMed]
- Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg 2001;25:891-7. [Crossref] [PubMed]
- Assié G, Antoni G, Tissier F, et al. Prognostic parameters of metastatic adrenocortical carcinoma. J Clin Endocrinol Metab 2007;92:148-54. [Crossref] [PubMed]
- Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol 2010;17:263-70. [Crossref] [PubMed]
- Polat B, Fassnacht M, Pfreundner L, et al. Radiotherapy in adrenocortical carcinoma. Cancer 2009;115:2816-23. [Crossref] [PubMed]
- Nakano R, Satoh D, Nakajima H, et al. Repeated resections for liver metastasis from primary adrenocortical carcinoma: A case report. Int J Surg Case Rep 2015;9:119-22. [Crossref] [PubMed]
- Gaujoux S, Al-Ahmadie H, Allen PJ, et al. Resection of adrenocortical carcinoma liver metastasis: is it justified? Ann Surg Oncol 2012;19:2643-51. [Crossref] [PubMed]
- Raff H, Shaker JL, Seifert PE, et al. Intraoperative measurement of adrenocorticotropin (ACTH) during removal of ACTH-secreting bronchial carcinoid tumors. J Clin Endocrinol Metab 1995;80:1036-9. [PubMed]
- Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 2007;356:2372-80. [Crossref] [PubMed]
- Peppa M, Pikounis V, Papaxoinis G, et al. Adrenocortical carcinoma secreting cortisol, androgens and aldosterone: a case report. Cases J 2009;2:8951. [Crossref] [PubMed]


