Three routine free flaps per day in a single operating theatre: principles of a process mapping approach to improving surgical efficiency
Introduction
Microsurgical based breast reconstruction using autologous tissue has become the standard of care across Europe and the United States, although access to free tissue transfer expertise can be variable. In areas where free tissue transfer is available, the number of women being offered this type of reconstruction is increasing, matching increases in the number of women being diagnosed with breast cancer who will require reconstruction. Furthermore, there has been a recent increase in the number of women requiring bilateral reconstructions, with a move towards bilateral risk reducing mastectomies. The reason for the increase in prophylactic mastectomies is only partially explained by the availability of BRCA-1 and -2 testing, but may also be due to an increased awareness of the quality of breast reconstruction and recent coverage of well-known media personalities having bilateral mastectomies.
There are many popular options available for free flap autologous breast reconstructions, including transverse rectus abdominis myocutaneous (TRAM) flaps (1), transverse upper gracilis (TUG) flaps (2), and profunda femoris artery perforator (PAP) flaps (3), however the deep inferior epigastric artery perforator (DIEP) flap remains the commonest and most accessible perforator based flap used in autologous breast reconstruction (4). We have developed our breast reconstruction service and attempted to streamline the process, in the belief that microsurgical breast reconstruction should be viewed as a routine procedure rather than a complex procedure, which takes up an entire day’s operating list as has been the case in the past. The current paper highlights our experience of breast reconstruction, and while it refers to DIEP flap based breast reconstruction, the principles can equally be applied to other types of free tissue transfer.
Methods and technique
A process mapping approach to free flap breast reconstruction is presented. The approach described has broken down the breast reconstruction procedure into several overlapping processes and describes the modifications taken to introduce efficiency savings at every step. Of note, this is “our” approach to process mapping, and while changes to the approach are inevitable between surgeons, the principles are presented for maximizing efficiency.
The first two processes do not specifically relate to operative timings, but if done efficiently, they contribute to reducing operative time.
Process 1—the initial consultations
The process mapping approach we adopt to streamline free flap based breast reconstruction begins from the time the patient is referred, having been given a diagnosis of breast cancer or has taken the decision for prophylactic mastectomy. Most often the patient will have been seen by her breast surgeon at a clinic distant to our hospital and is referred for plastic surgical opinion, at which stage we will first meet the patient at our next outpatient clinic. The time delay between referral and clinic appointment is usually a matter of days, and this first process in the patient’s reconstructive journey can be optimized to streamline clinic spaces available for these patients.
At this first point of patient contact, we discuss possible reconstructive options with the patient taking into account patient wishes, breast size, and available donor tissue and donor site morbidity. The aim by the end of the initial consultation is to formulate a reconstructive plan and offer appropriate informed consent. We aim for this initial consultation to be complete, so that the patient is ready to proceed with surgery and will not require further consultation until she is admitted for surgery. Given that there is a lot of information to be exchanged in this initial consultation and many women will have further questions that they may not have thought of during their consultation, a forum for further informal and formal discussions is essential, and we run weekly evening meetings to which all our pre-operative patients are invited. These are attended by specialist breast reconstruction nurses, past patients and medical staff intermittently. These consultations can also contribute directly to reducing operative time through proper documentation and consent, and can aid documentation as to examination findings for operative markings for surgery (and avoid perioperative changes to the operative plan).
Process 2—pre-operative assessment
All patients are seen pre-operatively by specialized nurses and anaesthetists familiar with free tissue transfer breast reconstruction to ensure they are fit for surgery, and nursing and anaesthetic plans are established early that may be specific to the patient. Pre-operative imaging with computed tomographic angiography (CTA) is performed, with a view to identifying the most suitable perforators and the most suitable hemi-abdomen for flap harvest. All aspects of the perforator course are assessed, with this “virtual surgery” able to preempt surgical dissection and reduce decision making intra-operatively (5-8). Upon admission to hospital, patients are also marked for surgery and perforator location confirmed using the hand-held Doppler probe.
Process 3—anaesthesia and turnaround time between patients
Time saving steps during the induction and maintenance of anaesthesia, while relevant, can only be employed if patient safety during anaesthesia can be maintained. For airway maintenance, we use a laryngeal mask as opposed to endotracheal intubation and use total intravenous anaesthesia rather than volatile gases for maintenance of anaesthesia. For our free flap procedures, we aim for the patient to be normotensive, normothermic and normovolaemic throughout the procedure.
Our experience with using the intra-operative oesophageal Doppler monitor for haemodynamic monitoring in free perforator flap surgery has been published previously (9), and in our experience, the placement of a central or arterial line can take up to 20 min which is compared to the 2 min taken for the siting of an oesophageal Doppler probe via the port on a laryngeal mask—a saving of 18 min. Further benefits of the oesophageal monitor are reduced fluid retention, reduced overall hospital stay and fewer post-operative complications, while offering all of the safety in haemodynamic monitoring offered by other more invasive means (see Figure 1).
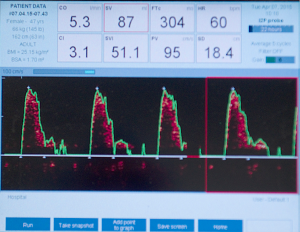
Our theatre turnaround time between patient entering the anaesthetic bay to being fully anaesthetized in theatre and fully prepped and draped ready for surgery has been reduced to a mean of 17 min (range, 14-25 min).
Process 4—breast reconstruction surgery
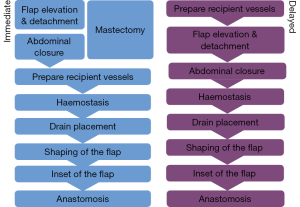
We use a two-team approach with one team harvesting the flap whilst the other simultaneously carries out the mastectomy. The most common reconstructive option we employ is the DIEP flap, with the TUG flap, PAP and lumbar artery perforator (LAP) flaps, also used for autologous reconstruction on occasion. Regardless of operative position, we always undertake both flap harvest and mastectomy simultaneously. The reconstructive team harvesting the flap consists of a plastic surgeon with an assistant (typically a trainee plastic surgeon or scrub nurse). The specific steps involved for both immediate and delayed DIEP based reconstruction are set out in Figure 2. When performing three consecutive free flap reconstructions in a single theatre, each step becomes time critical, as a delay at any point has a knock-on effect in terms of timing for the subsequent patients.
Mastectomy
The timing of mastectomy is out of the control of the reconstructive surgeon but working with a regular breast surgeon can reduce potential variability. If the patient requires sentinel lymph node biopsy and subsequent axillary node dissection, this stage will be prolonged; however, the flap can be completely harvested and the donor-site closed whilst the breast surgeon completes their part (described in more detail below).
Perforator selection
Perforator selection at the suprafascial plane is anecdotally the step that causes most anxiety and is the largest delay in efficiency, particularly for the less experienced perforator flap surgeon. With an increased use of preoperative imaging, we plan our perforator selection completely preoperatively, and can then aim immediately for the perforator vessel which is of largest calibre and most central to the flap, with almost no intraoperative delay in decision making at this step (10). Perforator choice is optimally achieved with an objective imaging modality such as magnetic resonance angiography (MRA) or CTA, with our preference CTA due to cost, availability and resolution. While we do not rely on the hand held Doppler signal to identify the largest perforator (as we have found this to be reliable only for determining the location of the perforators but not the calibre) we do use it adjunctively for location confirmation.
When carrying out unilateral beast reconstruction we will harvest the contralateral hemi-DIEP flap and move rapidly towards the midline where we expect the larger perforators to be located, safe in the knowledge that we have the other hemi-DIEP flap if we do not find suitable perforators where we would expect them to be. A common pitfall is to preserve more lateral perforators which tethers the flap laterally, limits the rate and range of medial dissection of the flap and increases the risk of damage to the poorly visualised medial perforators. Figure 3 highlights the sequence of perforator selection. If no single perforator is identified then we will on occasion take two smaller perforators and this choice is determined partly by the size of flap required which of course is dependent on breast size.
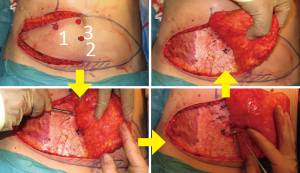
We do not expend time leaving the flap to perfuse on the perforator, nor dissect several perforators and place micro clamps on all but one perforator to check perfusion, if a perforator is visibly and palpably pulsatile we are confident it will have adequate perfusion pressure to supply the flap. Further we will always discard the portion of flap most distal to the perforator to minimise potential perfusion problems and any future fat necrosis.
Perforator dissection
The time variability of this step depends both on anatomical variability and surgeon experience. The preoperative CTA is useful here to provide a picture of the intramuscular course of the perforator as a paramedian perforator with a short intramuscular course is faster to dissect than one with a long intramuscular course. If we are dissecting out two perforators this will also increase the time taken and may require division of part of the rectus muscle or if there is a wide section of muscle between the two perforators we may divide one of the perforators from the main deep inferior epigastric artery and reconnect the two perforators on the back table with the operating microscope which allows us to preserve more of the rectus muscle to minimise any potential post-operative abdominal wall weakness.
Once the perforator is dissected free from the rectus muscle, we continue the dissection towards the pelvis and divide the pedicle just caudal to the limit of the inferior abdominal incision as excessive pedicle length is often unnecessary, particularly when anastomosing to the internal mammary vessels.
Flap shaping
Once the flap is elevated, there are several concurrent manoeuvres that can save considerable time. Much time is used inefficiently by delaying donor site preparation, donor site closure, flap shaping and preparation, and deepithelialisation. These steps, if done early, can optimise patient heat-loss from exposed deep tissues, can prevent pedicle damage from overzealous retraction if done later and can facilitate concurrent operating at this early stage in flap transfer. We achieve this by temporarily insetting the flap in the mastectomy defect and establishing the required flap volume and shape, and determining the size of the required skin paddle and area for deepithelialisation.
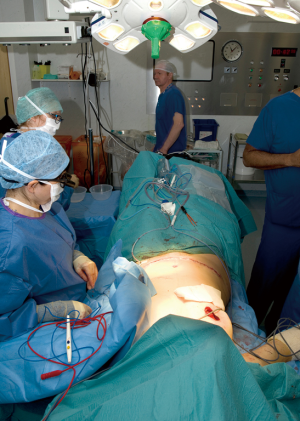
Two surgical teams are thus optimally utilized, with the abdominal donor site closed by one team while the other completes the mastectomy (see Figure 4), and after donor closure, one team shapes the flap while the other prepares recipient vessels. In doing this, the flap undergoes shaping and deepithelialisation (see Figure 5). At this point we will remove at least the Hartramp/Holm (1,11) zone 4 of the flap to minimise potential future fat necrosis and any more as necessary dependent on breast size. This part of the procedure can be carried out by an assistant whilst the recipient vessels are being prepared or by the primary surgeon if the mastectomy is still proceeding.
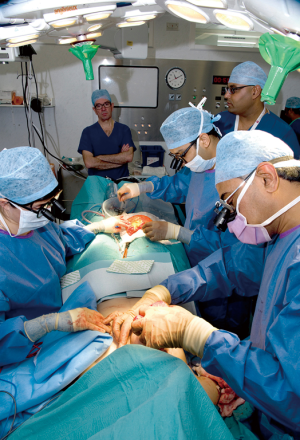
Recipient vessel preparation and anastomosis
Once the mastectomy is completed, an initial manoeuvre is to look for the second or third intercostal perforators, which is most often of sufficient calibre to allow a satisfactory anastomosis and obviates then need to dissect out the internal mammary vessels deep within the intercostals space and clearly avoids any rib resection. If the perforator has been damaged then our next choice is to explore the thoracodorsal axis which we access though a separate axillary incision. This is particularly useful after axillary dissection, as the entire subscapular axis is frequently exposed and requires minimal dissection. We then align the flap pedicle for anastomoses, with a hand sewing technique used for the artery and a micro-venous anastomotic coupler used for venous anastomosis. The efficacy and time saving benefits of which have been well documented (12,13).
Flap inset and abdominal closure
For abdominal closure we use an inlay Vicryl mesh (Ethicon, Wokingham, UK) placed under the rectus muscle and running two-layer non-absorbable sutures to the anterior rectus sheath. The upper abdominal flap is dissected to the costal margins centrally and closed with deep absorbable sutures then a single continuous barbed dermal suture (V-Loc, Covidien Mansfield, MA; or other) which is also time-saving in avoiding large numbers of deeper sutures. The flap is inset with several absorbable sutures to the chest wall superiorly and skin closed using several deep dermal absorbable sutures followed by a running barbed stitch.
Results
In a 12-month period, the senior author carried out 163 free flaps, both as primary operator and as an assistant to the primary operator. The multiple processes involved in DIEP flap based breast reconstruction are overlapping and varied depending on whether the breast reconstruction is immediate, delayed, unilateral or bilateral. Figure 6 shows the steps and the mean timings involved in an immediate unilateral DIEP flap breast reconstruction.
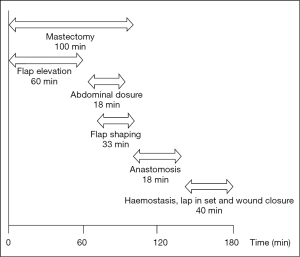
The mean operative time was 248 min, with the majority of cases completed such that 3 cases were completed within 12.5 h (between the hours of 08:30-21:00). The breakdown of times to facilitate these comprised anaesthetic time, flap harvest, micro-anastomotic times and flap inset and patient transfer. Anaesthetic times, comprising entry to the anaesthetic bay, line insertion and intubation and prepping and draping had a mean time of 17 min (range, 14-25 min). From the end of this “anaesthetic time”, flap raise time (comprising the time of knife to skin, until ischemic time begins) comprised a mean of 118 min (range, 60-237 min), ischaemic time (from the end of flap “flap raise time” until the end of anastomotic time—thus including the time for flap shaping and inset and vessel preparation) was 103 min (range, 45-220 min). The times for bilateral cases were somewhat longer, with combined flap raise time taking a mean of 173 min (range, 86-270 min) and ischaemic time a mean of 228 min (range, 120-450 min). The breakdown of ischaemic time to also include anastomotic time is presented in Tables 1,2. The additional 10 min over the combined means of each component equated to time for dressings and transfer out of theatre.
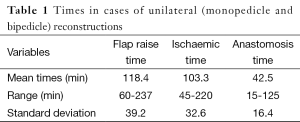
Full table
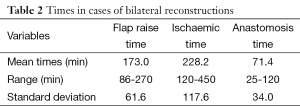
Full table
We have evolved the processes required so that there is as little unproductive time as possible and the sequence of events can be followed whether there is a single operator or whether there is an assistant involved. While the above times were recorded independently of simultaneous operating, the over-all times were routinely less than the combined totals of each component. Of note, in delayed reconstruction cases, if the operator is alone the sequence is to first carry out the flap dissection then to prepare the recipient site and vessels and inset the flap; if there are two surgeons then the flap and recipient vessels can be prepared be simultaneously.
In our series of DIEP flaps for breast reconstruction over a 12-month period, 84% were raised on a single perforator which was felt to be large enough to supply the whole flap. The remaining 16% were raised on two perforators when we had concerns that a single perforator would not be adequate. Looking at recipient sites for the vascular anastomosis, we used the 2nd intercostal perforator in 32% of cases, 3rd intercostal perforator in 43%, internal mammary vessels in 14%, thoracodorsal pedicle 5% and serratus anterior pedicle in the remaining 6%.
The increased efficiency and decreased operating times associated with this series was not associated with an increased complication rate in any recorded outcome measures. There was an overall 98.5% flap survival rate. This was in the context of a 2.5% early return to theatre rate, due to a combination of pedicle compromise and haematoma, and a 4% readmission to hospital rate (associated with mastectomy flap necrosis and flap necrosis in the majority of cases). These figures were the same as in the preceding period.
Discussion
Perforator based flaps are now the gold standard for breast reconstruction, minimising donor morbidity and providing adequate volumes of autologous breast like tissue. The reliability of the DIEP flap has been well established with many centres across the world quoting flap survival rates between 95-99% (14,15). Despite this, there remains the perception that free tissue transfer for breast reconstruction is a high risk, time consuming procedure with aesthetic outcomes not too dissimilar to implant based reconstruction such that it is not worth the extra time and effort. In this paper we have tried to show that it is possible to provide a high volume free flap based breast reconstruction service through a process-led approach to maximise time efficiencies at every step.
Others have described manoeuvres to increase surgical efficiency so that two DIEP flaps can be carried out in one day across two theatres (10) which included the use of venous coupler device, Cook-Swartz implantable Doppler devices, CT angiography and team work. We have taken this one stage further and regularly carry out three free flap reconstructions in a single theatre by streamlining the processes involved from the patient arriving in theatre, through their anaesthetic, their mastectomy and reconstruction to their recovery. Our regular theatre schedule is a 12-h day from 8.30 a.m. to 8.30 p.m. and we will routinely schedule two immediate unilateral reconstructions and a single unilateral delayed case in this time. As set out in Figure 6, our average time for an uncomplicated unilateral immediate reconstruction is 3 h so in a 12-h day, we have been able to operate on three patients with some flexibility in case one of the processes in the sequence takes longer than expected.
As patient awareness of breast reconstruction increases, as well as improvements in genetic testing and risk profiling for breast cancer, we are noticing an increase in the number of women referred for risk reducing mastectomies either at the same time as mastectomy for cancer or prophylactically. A combination of these factors has led to an increase in the number of bilateral breast reconstruction cases which clearly occupies more theatre time than a unilateral reconstruction. The trend towards a greater number of bilateral reconstructions is similar to recent data from the USA which found an increase from 3% in 1998 to 18% in 2007 (16), and this is likely to continue to increase. The ability to carry out more than one free flap breast reconstruction on a single operating list will become increasingly important if the demand for bilateral reconstructions continues to increase at the present rate.
Conclusions
The current paper demonstrates that it is possible to carry out three unilateral free flap breast reconstructions in one day in a single theatre. The safety of the patient remains of primary concern, followed by the importance of achieving sound oncological clearance and finally a pleasing aesthetic outcome with minimal donor site morbidity. In a healthcare environment increasingly sparse of resources however, health economics and operative efficiency are of increasing importance too. A process-mapped approach can contribute to operative efficiency.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 1982;69:216-25. [PubMed]
- Yousif NJ. The transverse gracilis musculocutaneous flap. Ann Plast Surg 1993;31:382. [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [PubMed]
- Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg 2012;129:16e-23e. [PubMed]
- Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16. [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [PubMed]
- Rozen WM, Ashton MW, Grinsell D, et al. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery 2008;28:306-13. [PubMed]
- Phillips TJ, Stella DL, Rozen WM, et al. Abdominal wall CT angiography: a detailed account of a newly established preoperative imaging technique. Radiology 2008;249:32-44. [PubMed]
- Figus A, Wade RG, Oakey S, et al. Intraoperative esophageal Doppler hemodynamic monitoring in free perforator flap surgery. Ann Plast Surg 2013;70:301-7. [PubMed]
- Acosta R, Enajat M, Rozen WM, et al. Performing two DIEP flaps in a working day: an achievable and reproducible practice. J Plast Reconstr Aesthet Surg 2010;63:648-54. [PubMed]
- Holm C, Mayr M, Höfter E, et al. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg 2006;117:37-43. [PubMed]
- Rozen WM, Whitaker IS, Acosta R. Venous coupler for free-flap anastomosis: outcomes of 1,000 cases. Anticancer Res 2010;30:1293-4. [PubMed]
- Grewal AS, Erovic B, Strumas N, et al. The utility of the microvascular anastomotic coupler in free tissue transfer. Can J Plast Surg 2012;20:98-102. [PubMed]
- Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg 2004;113:1153-60. [PubMed]
- Sailon AM, Schachar JS, Levine JP. Free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps for breast reconstruction: a systematic review of flap complication rates and donor-site morbidity. Ann Plast Surg 2009;62:560-3. [PubMed]
- Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol 2014;32:919-26. [PubMed]


