Laparoscopic and robotic adrenal surgery: transperitoneal approach
Introduction
Adrenalectomy has been an evolving surgical technique for the treatment of adrenal pathologies for more than a century. The first planned adrenalectomy was performed in 1914 by Perry Sargent (1) and since then, multiple changes in the surgical approaches to the adrenal gland have been developed. The advent of new technology and the desire of surgeons to reduce surgical morbidity, have contributed to the introduction of minimally invasive and remote-access adrenal surgery in the past decade. Gagner et al. (2) performed the first laparoscopic transperitoneal adrenalectomy (LA) in 1992. Since then, laparoscopy been regarded as the gold standard for the management of most adrenal surgical disorders. The wide acceptance of LA as a safe and effective procedure is primarily due to the improved patient outcomes such as shorter hospitalization, reduced pain and improved recovery. Furthermore, recent advances in minimal invasive surgery and increase in familiarity of the technique have challenged surgeons with laparoscopic skills to resect complex adrenal masses laparoscopically. Notwithstanding these benefits, LA has certain drawbacks including the 2-dimensional view, unstable camera platform, and poor ergonomics for the surgeon and rigid instrumentation.
Inspired by the drawbacks of laparoscopic surgery, robotic technology has been recently introduced as an armamentarium to minimal invasive adrenal surgery owing to the 3-dimensional view, wristed instrument, and stable camera platform (3). Theoretically, these advantages can help develop the LA procedure, thereby leading to improved perioperative and postoperative outcomes. Although there have been numerous reports demonstrating the safety and efficacy of robotic adrenalectomy (RA), the current drawbacks associated with RA include its cost, technical difficulties and the need of advanced training. In addition, robotic surgery is an advanced technology that requires a team with technical expertise to ensure successful accomplishment of the procedure.
The location of the adrenal glands, in the upper retroperitoneal space has led to the development of several surgical approaches including the anterior transperitoneal, lateral transperitoneal (LT) and the recently popularized posterior retroperitoneal (PR) which was first described by Mercan et al. (4). Proponents of each technique propose its superiority; however a consensus on the indications and choice of approach has not yet been reached. Among these techniques the most frequently preferred is LT adrenalectomy, since it provides a wider operative field, visibility and anatomical familiarity for most surgeons (5-7). The aim of this review is to summarize the available data on laparoscopic and RA via the transperitoneal approach in terms of indications, perioperative outcomes, cost and recent developments.
Indications and patient selection
Current indications for transperitoneal adrenalectomy are similar for both laparoscopic and robotic approaches, and include all benign functional adrenal tumors, benign non-functional tumors ≥4 cm or those demonstrating significant growth on follow-up CT scan, as well as adrenal metastases in selected patients with soft-tissue or solid-organ primary tumors, usually in the setting of mono or oligometastatic disease (8-10). Also the larger working space and the familiarity of landmarks associated with the LT technique make it more favorable for larger, unilateral tumors, in which there is a small retroperitoneal space or previous retroperitoneal kidney surgery. Furthermore, it offers a lesser burden in events that conversion to an open transperitoneal approach is required. An open technique is the management of choice for lesions that are highly suspicious for adrenocortical cancer based on preoperative clinical, biochemical and imaging findings (11-13). In scenarios where evidence suggestive of gross extra-adrrenal invasion during a laparoscopic/robotic-assisted adrenalectomy exists, early conversion to an open approach prior to any significant dissection is warranted to ensure an effective execution of an oncologically sound procedure consisting of en-bloc resection of the tumor, regional lymphadenopathy and removal of contiguously involved organs. In our institution we prefer LT adrenalectomy for tumors >6 cm. If the tumor size is <6 cm, the distance between the skin and Gerota’s space is not long (generally <7 cm) and the 12th rib is rostral to the renal hilum, we carry out the PR technique.
Operative technique
Patient preparation, positioning, and port placement are the same for both laparoscopic and robotic techniques. The patient is placed in a lateral decubitus position (Figure 1). Adrenalectomy is generally performed using four ports. It is important to be able to configure the trocar placement such that to give the 1st assistant an access to use the suction-irrigator device and possibly a clip applicator. Generally, this is the most medial port for right-sided adrenalectomy and the most lateral port for left-sided procedures. In obese or small patients, it might be necessary to change the location of the 1st assistant port, depending on the anatomy.
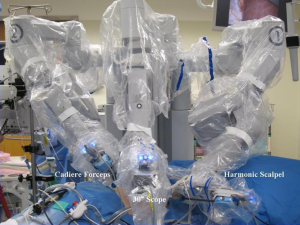
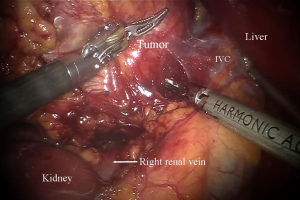
For the robotic technique, we prefer to use the bipolar Cadiere forceps, robotic harmonic scalpel, and a 30-degree down scope to do the dissection (Figure 2). We do the initial exposure including division of the right triangular ligament and splenorenal ligament laproscopically, followed by laparoscopic ultrasound, before we dock the robot. We use 8 mm instruments for the lateral transabdominal technique. The communication with the anesthesia team is very important for a fast docking. The table might need to be reversed in order not to block the docking of the robot. Based on the patient’s anatomy, we rotate the table clockwise in order to match the angle of dissection with the docking of the robot. The robot is docked into position coming from the ipsilateral shoulder of the patient and connected to the robotic trocars. On the right side, the 1st assistant retracts the liver and suctions using a long suction-irrigator tip. On the left side, the 1st assistant, similarly, provides suctioning and counter-traction with the same instrument. The adrenal vein is divided using the Harmonic scalpel if <4 mm and using clips if larger (Figure 3). We prefer metallic clips placed by the 1st assistant, but robotic locking clips may also be used. After the adrenalectomy is complete, the robot is undocked, and the gland is removed using a specimen retrieval bag. After the operative site is irrigated and suctioned, trocars are removed. Morcellation may be required if the specimen is large (e.g., >3 cm). Hemostasis is achieved laparoscopically and confirmed after desufflation and reinsufflation. Fascia is closed for both 12 mm port sites.
Outcomes
Over the past decade, the safety and feasibility of RA have been reported by several studies. A series of 30 RAs performed by three surgeons at a single institution was reported for the first time by Winter et al. in 2006. The median operative time was 185 minutes. The operative time for one of the surgeons improved significantly over time, at a rate of 3 min per case. No conversions to laparoscopic or open surgery were seen. A postoperative morbidity rate of 7% was encountered and this included; prolonged ileus (one case) and hypoxia (one case). There was no perioperative mortality. The median hospitalization was 2 days (14).
In 2008, Brunaud et al. prospectively evaluated 100 patients who had undergone robotic transperitoneal unilateral adrenelectomy. There were no mortalities and morbidity rate was 10%. The morbidities reported were mainly wound infection (n=2), facial edema (n=1), pneumonia (n=3) urinary tract infection (n=1), postoperative anemia (n=1) and hematoma (n=1). The authors reported a conversion rate of 4% to laparoscopy and 1% to laparotomy. The mean operative time was 99±35 min. The mean operative time decreased by 5 min for junior surgeons in every 10 cases whereas senior surgeons gained 2 min in every 10 cases. They concluded that although the robotic approach was not safer and cheaper than laparoscopic surgery, it provided significant advantages to the surgeon, such as more ergonomics and better image quality (15).
Giulianotti et al. later reported a series of 42 patients who underwent transabdominal lateral RA for various unilateral benign and malignant adrenal tumors. They had no conversions, but one intraoperative complication due to capsular tear in a case of 6 cm pheochromocytoma. The authors advocated that robotic adrenal surgery could be beneficial for patients with higher BMI and large tumors and that it could be a valid option in high volume centers in terms of outcomes and feasibility (16).
A group from a university hospital in Sweden, also reported their experience with 100 patients who had undergone robotic LT adrenalectomy. They had a 7% conversion rate where all the cases were converted to open surgery. They demonstrated a correlation between the BMI of the patient and the weight of the specimen in converted cases (P=0.047 and P=0.066, respectively). They had no mortalities reported and 13 patients had minor postoperative complications. Interestingly, findings from this study showed that, patients operated for left-sided tumor have a significantly higher postoperative complication rate than right-sided tumors (18% vs. 5%, respectively) (17).
Our initial RA experience consisted of 50 patients. The procedure was performed for both LT and PR approaches. There was no difference when we compared the robotic with the laparoscopic groups regarding demographics, tumor type, and body mass index. For the LT approach, despite larger tumor size in the robotic vs. the laparoscopic group (4.7 vs. 3.8 cm), the operative times were similar (168 vs. 159 min). There was no difference between the two approaches regarding the time spent for the individual steps of the operation. In the PR approach, with similar tumor sizes (2.7 vs. 2.3 cm), operative time was equivalent (166 vs. 170 min). Shorter time for hemostasis was significant in the robotic group. For laparoscopic and robotic procedures, the morbidity was 10% and 2%, respectively (18).
Recently the soaring interest for RA has led to reports from several studies comparing RA to LA in terms of perioperative outcomes. Advocates of the robotic approach report its merits over laparoscopy owing to the better surgical ergonomics and 3-dimensional view, however, a consensus on whether one approach is superior over the other hasn’t been reached yet since a large scale randomized controlled trail comparing both approaches is lacking in the current literature.
The first randomized trial comparing laparoscopic to RA using a LT approach was published by Morino et al. (19) in 2004. The study which randomly assigned 20 patients with benign adrenal lesions to each arm with exclusion criteria including bilateral lesions and tumors >10 cm showed that RA was associated with longer operative times (169 vs. 115 min), increased perioperative morbidity (20% vs. 0%), and higher total costs ($3,467 vs. $2,737, excluding initial robot cost) relative to the laparoscopic approach.
Brunaud and colleagues evaluated the quality of life of patients’ after RA or LA. Overall, 21 patients were assessed once preoperatively and twice postoperatively on validated measures of physical pain, physical functioning, emotional functioning, and anxiety. RA was compared with LA across ten different measures at three different time points. Only one significant difference between the two groups was noted. At 6 weeks postoperative, the robotic group had a significantly increased score of “role limitations due to emotional problems”. There were no other significant differences. The authors concluded that, perioperative quality of life is not a justifiable parameter on which to base promotion of RAs (20).
Subsequently, a retrospective chart review of patients who received either robotic (n=50) or laparoscopic (n=59) unilateral LTA by the same authors reported that the robotic approach was associated with less intraoperative blood loss although longer operative times overall compared with the laparoscopic approach (21). As expected, a learning curve of 20 more cases on the robotic platform nullified this difference in operative time. In addition, a subset analysis showed that laparoscopic adrenalectomy (LA) was associated with longer operative times in patients with larger tumors (>5.5 cm) as well as in those with a BMI ≥30.
We have reported our experience with 63 patients receiving either laparoscopic (n=32) or robotic PRA (n=31). Tumor size, blood loss, and hospital stay were similar between the two groups, as were overall skin to skin operative times. After an initial learning curve of 10 cases, however, operative times were significantly shorter in the robotic group (139 vs. 167 min), inclusive of robotic docking times, which ranged from 5-30 minutes. Pain scores on postoperative day 1 were lower in the robotic group than in the laparoscopic group, which was attributed to the potentially shorter operative time, and less pressure on incisions as a result of fewer instrument changes and articulating instrumentation (22).
A current meta-analysis of 600 patients from eight retrospective studies and one RCT undergoing either robotic (n=277) or laparoscopic (n=323) adrenalectomy, showed no significant difference in operative time, conversion rate, or postoperative complications was observed between the two groups, although there was a significantly shorter hospital stay (WMD—0.43 days) and estimated blood loss (WMD—18.2 mL) in the robotic group (23).
In 2008, a case-control study comparing robotic and conventional unilateral laparoscopy included 48 patients that underwent robotic uniLT adrenalectomy at a single institution. The patients were matched according to tumor size, surgeon’s experience, and patient BMI with 48 LA patients. Findings from this study did not show any statistically significant differences between RA and LA groups with regard to demographic features, endocrine disorders, side of lesion and tumor, patients BMI and surgeon’s experience (senior vs. junior). Perioperative outcomes were similar in both groups. Mean operative time (87 vs. 86 min), morbidity (10% vs. 17%), conversion (5% vs. 4%), and mortality (0% vs. 0%). In agreement with previously reported studies, the mean hospital stay was shorter after RA than after LA (6.1 vs. 6.8 days; P=0.02) (24).
Notwithstanding the increase in interest for RA recently, the technology still needs ample time to develop. Large volume centers are needed to determine what differences, if any, exist between robotic and conventional LA.
Laparoscopic and robotic surgery for large adrenal tumors
Due to the technical difficulties, higher risk of complications and concerns of malignancy, minimally invasive resection of large adrenal tumors can be challenging (25,26). The transperitoneal approach provides greater exposure for the resection of larger adrenal tumors. (>5 cm). Lezoche et al. compared the retroperitoneal approach to the anterior or LT approaches for the resection of larger tumors. In their study, the authors found that, the transperitoneal approaches (anterior and lateral) are better for the resection of larger adrenal masses and this was evidenced by a statistically significant difference in mean gland diameter (27).
Resection of adrenal masses larger than 14 cm in diameter through a restricted retroperitoneal space can be challenging. This has been one of the major drawbacks of the posterior approach (28). In a study comparing the LT, anterior transperitoneal and retroperitoneal approaches, although intraoperative time did not differ significantly among the three approaches, a significantly shorter operative time for the LT approach was reported. In the same study, adrenal masses were significantly larger in subjects in whom the transperitoneal approach was preferred. The authors concluded that, for tumors >5 cm, the transperitoneal technique should be the preferred approach (29).
Our group previously reported a comparative study of robotic vs. laparoscopic resection of large adrenal tumors. The study included 25 patients with 25 tumors in the robotic and 38 patients with 39 tumors in the laparoscopic group. There was similarity in both groups in terms of tumor size (6.5 vs. 6.2 cm, respectively). A shorter operative time was observed in the robotic versus laparoscopic group (159 vs. 187 min, respectively); while, estimated blood loss was similar. Conversion rates were as follows; 4% vs. 11% in the robotic and laparoscopic group respectively. Hospital stay was shorter for the robotic group (1.4 vs. 1.9 days, respectively) and there was no morbidity in the robotic group. A 2.7% morbidity rate was detected in the laparoscopic group. Findings from our study showed that the use of the robot shortened operative time and decreased the rate of conversion to open for adrenal tumors larger than 5 cm (30).
Laparoscopic and robotic adrenalectomy (RA) in pediatric patients and pregnant individuals
The role of minimally invasive approaches for adrenalectomy in pediatric and pregnant patients is poorly defined. Reports on the applications of laparoscopic and robotic assistance for this group of patients are few in the literature. Existing studies have shown the transperitoneal approach to have considerable benefits including a larger view of entire abdominal cavity and excellent exposure of both adrenal glands and surrounding structures (31).
Eassa et al. reported the outcomes of LA in eight patients with different adrenal tumors including aldosteronoma in three, virilising tumor in two, non-functioning tumor in one, Cushing’s syndrome in one and pheochromocytoma in one. The mean operative time was 60 min, with minimal intraoperative blood loss. Similar studies (32,33) have also reported the safety and feasibility of the procedure in the pediatric population.
In a series of 134 robotic pediatric surgical procedures, including adrenalectomy in one patient, Alqahtani et al. reported that robot-assisted surgery appeared to be safe and feasible for pediatric patients as well. The role of robotic partial adrenalectomy as a safe procedure providing precise dissection in a pediatric patient with VHL has also been described (34). A different study about the first pediatric robotic series in Spain concluded that, the learning curve of robotic surgery was shorter than the conventional laparoscopic surgery and the main obstacle in children was the smaller size of the operative field (35).
Podolsky et al. operated on a patient diagnosed with right-sided pheochromocytoma at 21 weeks of gestation through a LT approach with no postoperative complications (36). The authors advocated robotic adrenal surgery as a safe option for pregnant women since it provides a better vision and facilitates dissection in a limited space due to pregnancy.
Cost effectiveness
As the need to reorient health care around value for patients has now become an intrinsic motivation in the practice of medicine, further development of robotic adrenal surgery may be limited unless improved quality focusing on patient based outcomes can be used to justify the generally higher costs of this technology. The costs of robotic systems are fixed with prices ranging between USD 1 million and USD 2.5 million per unit (37).
Higher costs are also generated from related periodic maintenance, prolonged operative time, and associated facility and staffing fees. In the context of growing health costs in the United States, this represents a major concern at the national policy level. In a 2005 study by Bodner et al., RA was estimated to be 1.5 times more costly than traditional LA (38).
Similarly, a prospective evaluation of 100 robotic cases by Brunaud et al. showed that, RA was 2.3 times more expensive than LA (Euro 4,102 vs. Euro 1,799). The authors however noted a decrease in the calculated cost difference as the number of robotic cases increased and when depreciation of the robotic system and laparoscopic equipment was distributed over 10 years instead of 5 years (15).
A retrospective analysis of all cost studies of robot-assisted procedures published since 2005 by Barbash et al. found that, on average, across the full range of 20 types of surgery for which studies exist, the additional variable cost of using a robot-assisted procedure was about $1,600, or about 6% of the cost of the procedure in 2007. For unilateral adrenalectomies, the additional cost of using a robot was estimated from 1,400 to 2,900 USD, or about 10% to 20% of the cost of that procedure (37).
The adoption and use of new technologies, even when insurers do not explicitly provide reimbursement is an undeniable role of an efficient health care system. Efficient health systems should enhance the ability of medical professionals and their patients to make informed choices. Currently, intuitive surgical is the sole producer of robotic surgical devices; however in the future, prices are expected to decline if there is more competition in the market for machines or related consumables.
Conclusions
The laparoscopic technology has presented new frontiers to adrenal surgery thus enabling modification and refinement of established conventional procedures. The integration of robots into adrenal surgery has made it feasible to offer alternatives to patients requiring surgical treatment of adrenal disorders. Although there are many questions regarding its cost, training and efficiency compared to the laparoscopic approach, in the setting of our evolving value-based health care system, these limitations must be overcome quickly to merit continued development of these surgical techniques.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Welbourn RB. The adrenal glands. In: Welbourn RB, Friesen SR, Johnston ID, editors. The History of Endocrine Surgery. New York: Praeger, 1990:147-216.
- Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [PubMed]
- Horgan S, Vanuno D. Robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2001;11:415-9. [PubMed]
- Mercan S, Seven R, Ozarmagan S, et al. Endoscopic retroperitoneal adrenalectomy. Surgery 1995;118:1071-5. [PubMed]
- Li QY, Li F. Laparoscopic adrenalectomy in pheochromocytoma: retroperitoneal approach versus transperitoneal approach. J Endourol 2010;24:1441-5. [PubMed]
- Rubinstein M, Gill IS, Aron M, et al. Prospective, randomized comparison of transperitoneal versus retroperitoneal laparoscopic adrenalectomy. J Urol 2005;174:442-5. [PubMed]
- Kang WH, Kim BS, Choi YB. Comparison of Laparoscopic Transperitoneal Versus Retroperitoneal Adrenalectomy. J Korean Soc Endosc Laparosc Surg 2010;13:22-5.
- Vazquez BJ, Richards ML, Lohse CM, et al. Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg 2012;36:1400-5. [PubMed]
- Howell GM, Carty SE, Armstrong MJ, et al. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol 2013;20:3491-6. [PubMed]
- Zeiger MA, Thompson GB, Duh QY, et al. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract 2009;15:1-20. [PubMed]
- Stefanidis D, Goldfarb M, Kercher KW, et al. Guidelines for the Minimally Invasive Treatment of Adrenal Pathology. Available online: http://www.sages.org/publications/guidelines/guidelines-for-the-minimally-invasive-treatment-of-adrenal-pathology/
- Berruti A, Baudin E, Gelderblom H, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii131-8. [PubMed]
- Henry JF, Peix JL, Kraimps JL. Positional statement of the European Society of Endocrine Surgeons (ESES) on malignant adrenal tumors. Langenbecks Arch Surg 2012;397:145-6. [PubMed]
- Winter JM, Talamini MA, Stanfield CL, et al. Thirty robotic adrenalectomies: a single institution’s experience. Surg Endosc 2006;20:119-24. [PubMed]
- Brunaud L, Ayav A, Zarnegar R, et al. Prospective evaluation of 100 robotic-assisted unilateral adrenalectomies. Surgery 2008;144:995-1001. [PubMed]
- Giulianotti PC, Buchs NC, Addeo P, et al. Robot-assisted adrenalectomy: a technical option for the surgeon? Int J Med Robot 2011;7:27-32. [PubMed]
- Nordenstrom E, Westerdahl J, Hallgrimsson P, et al. A prospective study of 100 robotically-assisted laparoscopic adrenalectomies. J Robotic Surg 2011;5:127-31.
- Karabulut K, Agcaoglu O, Aliyev S, et al. Comparison of intraoperative time use and perioperative outcomes for robotic versus laparoscopic adrenalectomy. Surgery 2012;151:537-42. [PubMed]
- Morino M, Benincà G, Giraudo G, et al. Robot-assisted vs laparoscopic adrenalectomy: a prospective randomized controlled trial. Surg Endosc 2004;18:1742-6. [PubMed]
- Brunaud L, Bresler L, Zarnegar R, et al. Does robotic adrenalectomy improve patient quality of life when compared to laparoscopic adrenalectomy? World J Surg 2004;28:1180-5. [PubMed]
- Brunaud L, Bresler L, Ayav A, et al. Robotic-assisted adrenalectomy: what advantages compared to lateral transperitoneal laparoscopic adrenalectomy? Am J Surg 2008;195:433-8. [PubMed]
- Agcaoglu O, Aliyev S, Karabulut K, et al. Robotic vs laparoscopic posterior retroperitoneal adrenalectomy. Arch Surg 2012;147:272-5. [PubMed]
- Brandao LF, Autorino R, Laydner H, et al. Robotic versus laparoscopic adrenalectomy: a systematic review and meta-analysis. Eur Urol 2014;65:1154-61. [PubMed]
- Brunaud L, Reibel N, Ayav A. Pancreatic, endocrine and bariatric surgery: the role of robot-assisted approaches. J Visc Surg 2011;148:e47-53. [PubMed]
- Boylu U, Oommen M, Lee BR, et al. Laparoscopic adrenalectomy for large adrenal masses: pushing the envelope. J Endourol 2009;23:971-5. [PubMed]
- Sharma R, Ganpule A, Veeramani M, et al. Laparoscopic management of adrenal lesions larger than 5 cm in diameter. Urol J 2009;6:254-9. [PubMed]
- Lezoche E, Guerrieri M, Feliciotti F, et al. Anterior, lateral, and posterior retroperitoneal approaches in endoscopic adrenalectomy. Surg Endosc 2002;16:96-9. [PubMed]
- Bonjer HJ, Lange JF, Kazemier G, et al. Comparison of three techniques for adrenalectomy. Br J Surg 1997;84:679-82. [PubMed]
- Suzuki K, Kageyama S, Hirano Y, et al. Comparison of 3 surgical approaches to laparoscopic adrenalectomy: a nonrandomized, background matched analysis. J Urol 2001;166:437-43. [PubMed]
- Agcaoglu O, Aliyev S, Karabulut K, et al. Robotic versus laparoscopic resection of large adrenal tumors. Ann Surg Oncol 2012;19:2288-94. [PubMed]
- Sukumar S, Jadhav S, Nair B, et al. Laparoscopic adrenal surgery in children: Lessons from a single centre experience. J Minim Access Surg 2011;7:141-4. [PubMed]
- Heloury Y, Muthucumaru M, Panabokke G, et al. Minimally invasive adrenalectomy in children. J Pediatr Surg 2012;47:415-21. [PubMed]
- Eassa W, El-Sherbiny M, Jednak R, et al. The anterior approach to retroperitoneoscopic adrenalectomy in children: technique. J Pediatr Urol 2012;8:35-9. [PubMed]
- Alqahtani A, Albassam A, Zamakhshary M, et al. Robot-assisted pediatric surgery: how far can we go? World J Surg 2010;34:975-8. [PubMed]
- Marhuenda C, Giné C, Asensio M, et al. Robotic surgery: first pediatric series in Spain. Cir Pediatr 2011;24:90-2. [PubMed]
- Podolsky ER, Feo L, Brooks AD, et al. Robotic resection of pheochromocytoma in the second trimester of pregnancy. JSLS 2010;14:303-8. [PubMed]
- Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med 2010;363:701-4. [PubMed]
- Bodner J, Augustin F, Wykypiel H, et al. The da Vinci robotic system for general surgical applications: a critical interim appraisal. Swiss Med Wkly 2005;135:674-8. [PubMed]


