
The venous anatomy of the abdominal wall for Deep Inferior Epigastric Artery (DIEP) flaps in breast reconstruction
Introduction
The venous anatomy of the anterior abdominal wall differs from its arterial counterpart, with a dominant superficial venous drainage despite a dominant deep arterial supply. This inherent conundrum has had profound influences on the fate of flaps based on the abdominal wall integument, such as the deep inferior epigastric artery perforator (DIEP) flap, the transverse rectus abdominus myocutaneous (TRAM) flap and the superficial inferior epigastric artery (SIEA) flap. In two previous studies of the venous system of the DIEP flap for breast reconstruction, we were able to highlight the importance of the venous anatomy in flap success (1,2). In one of these studies, the clinical importance of considering both the superficial and deep venous drainage systems of the abdominal wall was highlighted (Figure 1) (2). In the other study, a cadaveric study was used to demonstrate the venous anatomy of the abdominal wall, and this led the way to clinical studies for highlighting this anatomy in-vivo (Figures 2,3,4) (1). The current review, including all of the clinical context, is based on that cadaveric study (1), and we offer a further review of the literature to highlight additions to our knowledge, including advanced imaging modalities with computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), as well as clinical findings of anatomical variations in the venous anatomy.
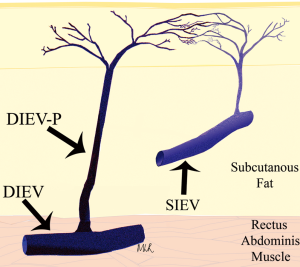
The importance of this venous anatomy to this flap, the deep inferior epigastric artery (DIEA) perforator flap, is essential. The DIEP flap has been widely shown to have a low incidence of complications, contributing to its popularization for use in breast reconstruction (3-6). While the arterial anatomy of the abdominal wall forming the basis for supply to this flap has been widely described in the literature, the venous anatomy has not received an equal appraisal (7,8). Despite this, venous problems continue to be the more frequently encountered vascular complications seen, with Blondeel (9) reporting a series of DIEA perforator flaps with insufficient venous drainage requiring re-operation, and this having been echoed in subsequent studies (9-11).
Many authors have since offered varying means to augmenting or supercharging the venous drainage of congested or compromised flaps. The methods used to augment this venous drainage have included the use of additional venae comitantes of the ipsilateral DIEA (12,13), the venae comitantes of the contralateral DIEA (14), through the ipsilateral superficial inferior epigastric vein (SIEV) (10,11,15), and the contralateral SIEV (16). In understanding the cause for venous problems in these flaps, Carramenha e Costa et al. undertook an anatomical study using corrosion casts and dye injection to describe the venous architecture of the abdominal wall integument, and demonstrated venous drainage through both superficial and deep venous systems (17). Subsequent studies have explored the use of plain-film angiography to evaluate this anatomy (18-20), and a recent anatomical study utilized cadaveric computed tomographic angiography (CTA) to evaluate the venous anatomy (21). With the increasing use of preoperative CTA, the ability to analyse the in-vivo venous architecture of the abdominal wall has become possible.
Previous cadaveric anatomical studies
In our previous cadaveric anatomical studies, we undertook contrast venography and dissection studies on 8 whole fresh cadaveric abdominal wall specimens. The cadavers spanned a wide range of body habitus types, and the cadaveric age ranged from age 50-85. No cadavers had undergone previous abdominal surgery. Six of these specimens were archival studies, utilized in previous studies of the venosomes of the body (20,22). In each case (both current and archival studies), the abdominal wall integument was harvested from its respective cadaver, in anticipation of contrast radiographic studies. A contrast mixture was made, comprising heated normal saline (to 50 oC), 10% w/v of commercial grade (96% pure) lead oxide as an orange powder and 10% w/v gelatine injection (23). The deep inferior epigastric vein (DIEV) and SIEV were then identified and cannulated, with each vessel injected with the contrast media. Each specimen underwent venography with plain-film radiography (Fuji Computed Radiography Processor, Model CR-IR 357, Fuji Film Corporation, Tokyo, Japan). In two specimens, periumbilical dissection was undertaken in order to identify veins for cannulation, with a view to filling veins that may have not filled with contrast initially due to the presence of valves. In these cases, repeat venography was undertaken.
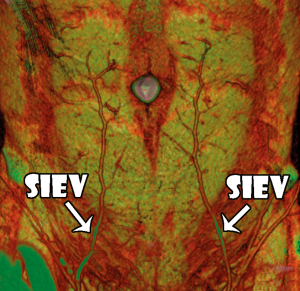
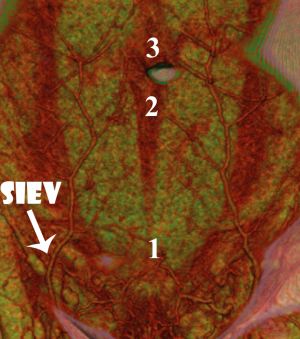
We identified that the SIEV was found to consistently lie superficial to Scarpa’s fascia (all 16 sides), to have a single (87.5% of sides) or bifurcating (12.5% of sides) trunk below the umbilicus, and to routinely overlie the rectus abdominis muscle at the level of the arcuate line. A large (>1 mm) medial branch was distributed that crossed the midline in 15 of 16 sides. While this crossover point was routinely at the level of the arcuate line, in some cases there were additional midline cross-over points seen: immediately infraumbilically and immediately supraumbilically (Figure 5). Inferiorly, the SIEV coursed through the superficial inguinal lymph nodes to drain into either the superficial femoral vein (62.5% of sides), the long saphenous vein (12.5% of sides) or the saphenous bulb (25% of sides). The mean diameter of the SIEV at its termination was 2.3 mm (range, 1.8-3.3 mm). It received tributaries throughout its course, including the superficial circumflex iliac vein (SCIV) and/or the superficial external pudendal vein (SEPV). The SIEV distributed multiple deep branches throughout its course, which comprised the venae commitantes of DIEA perforators. These perforator veins were numerous, and primarily showed a periumbilical distribution. They perforated the anterior rectus sheath to drain into the venae commitantes of the DIEA.
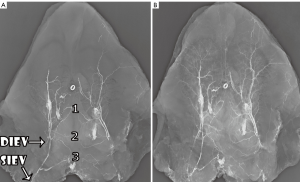
The venae commitantes of the DIEA ran alongside the major branches of the DIEA within or deep to the rectus abdominis muscle. Communications between the two venae commitantes were evident throughout their course, and below the arcuate line, the DIEA and its venae commitantes turned laterally to encroach upon the femoral vein, and the venae commitantes united to form a single DIEV. The mean diameter of the DIEV at its termination was 3.2 mm (range, 2.3-3.9 mm). Of note, the mean distance between the SIEV and the superficial inferior epigastric artery (SIEA) at the level of the inguinal ligament was 2.9 cm, while the mean distance between the DIEA and DIEV at this level and throughout their course was <0.5 cm.
During radiographic studies, both the SIEV and DIEV were incompletely filled with contrast upon direct injection at their terminations, suggesting the presence of valves orientated toward a caudal venous flow in both systems. In two of the eight cadaveric specimens, microsurgical dissection was undertaken to identify a periumbilical venous perforator, which was injected with contrast bidirectionally with a small calibre injecting needle. In these cases, contrast was seen both macroscopically and radiographically to flow into the deep system but not the superficial system, demonstrating the presence of valves in these perforators directing flow from superficial to deep.
In-vivo (clinical) anatomical studies
We undertook further anatomical studies in the clinical setting, with a cohort of 100 patients (200 hemi-abdominal walls) undergoing DIEP flaps for breast reconstruction included in the study. All imaging was performed between July 2006 and October 2008. Patients were recruited at a single institution, with institutional ethics approval, and no patients were excluded from the study. Patients were all female, were of a range of body habitus types, and were between 35-68 years of age. All imaging was performed at a single institution, using a 64 slice multi-detector row CT scanner (Siemens Medical Solutions, Erlangen, Germany), with 100 mL of intravenous contrast (Omnipaque 350; Amersham Health, Princeton, USA). CTA images were reformatted into maximum intensity projection (MIP) and 3-dimensional volume rendered technique (VRT) images using commercially available software (Siemens Syngo InSpace; Version: InSpace2004A_PRE_19, Pennsylvania, USA). Thin axial slices were used for all vessel measurements, with calibre measurements given as internal diameters. The location, size and course of the DIEV, SIEV and their communications were recorded.
We found that the superficial venous system and its communications with the deep venous system were readily demonstrated on CTA imaging (Figure 2), with characteristics of the venous system able to be recorded as with the cadaveric study. In all cases, the SIEV was found to consistently lie superficial to Scarpa’s fascia (all cases), to have a single (82% of sides), bifurcating (17% of sides) or trifurcating (1%) trunk below the umbilicus, and to supply a large (>1 mm) medial branch that crossed the midline in 86% of cases. This medial branch routinely crossed below the level of the arcuate line. In some cases, there were additional midline cross-over points seen: immediately infraumbilically and immediately supraumbilically (Figure 3). The SIEV drained into either the superficial femoral vein (41% of sides), the long saphenous vein (9% of sides), the saphenous bulb (49% of sides) or the SEPV (1%). The mean diameter of the SIEV at its termination was 2.5 mm (range, 1.8-5.2 mm). It was seen to receive numerous tributaries, including the superficial circumflex iliac vein (SCIV) and/or the superficial external pudendal vein (SEPV).
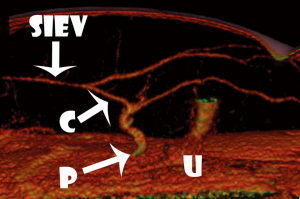
While the SIEV was shown to distribute deep branches which perforated the anterior rectus sheath to drain into the venae commitantes of the DIEA, these were not visible in all cases or for all perforators. This communication between deep and superficial venous systems was thus often too small to see with CTA. A communication between the SIEV and individual perforator veins was only identified in 90% of cases, and for only 1-3 perforators per patient amongst these patients (Figure 4).
Clinical outcome studies of venous drainage
In order to assess the role of dominance between the deep and superficial venous systems, as well as to assess the need for single versus double venous outflow routes in DIEP flaps, we undertook a retrospective study for patients having undergone DIEP flap breast reconstructions during the period of January 2000 to September 2008. This was a consecutive series, with all operations undertaken by a single reconstructive surgical unit, of four core surgeons. The only exclusion criterion was flaps that were supplied by more than one artery (stacked or bipedicled flaps). All flaps were fasciocutaneous, included no rectus muscle, and were raised on a single DIEA. Recorded data comprised patient demographics, operation details, complications, implementation of secondary venous outflow routes and details of the vascular basis for flap supply and drainage. Patients were stratified into two groups according to the number of veins used for venous drainage (one versus two). The decision to use an alternative (secondary) source of venous drainage was made based upon individual surgeon preference, with factors influencing this decision including a good match of two donor and recipient veins, the presence of a subjectively enlarged (greater than 1.5 mm) SIEV, a subjectively engorged (tense and dilated) SIEV, or in the presence of frank venous congestion during flap harvest or flap insetting (where pedicle flow continuity was confirmed to be present). The donor vessel of choice was the SIEV, in order to achieve venous flow through both deep and superficial venous territories, with a second DIEV (DIEA concomitant vein) as an alternative option. The contralateral SIEV was the preferred choice of vessel (97% of cases), however where inappropriate (inadequate size or absent vessel, or in bilateral reconstructions), the ipsilateral SIEV was used (3% of cases). Where an SIEV was used, the cephalic vein was used as the recipient vessel of choice, harvested through a small incision in an anterior axillary skin crease with minimal operative time or scarring (Figures 6,7,8). The full description of the technique is in the sub-section below. The use of the cephalic vein as a recipient vessel as described, and the use of anastomotic devices that achieve fast anastomotic times (either ‘Anastoclip’ Vascular Closure Staples (VCS) micro-staple clips or a microvascular anastomotic coupling device, allowed us to perform a second venous anastomosis with no increase in operative time. Our use of these anastomotic procedures has been described previously (24), and it should be noted that these occurred more frequently in the latter part of the series, and thus a learning curve is certainly an important consideration in evaluating surgical times.
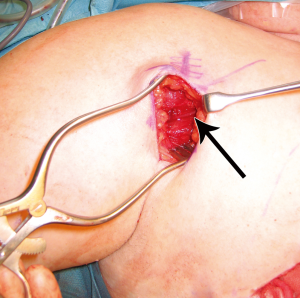
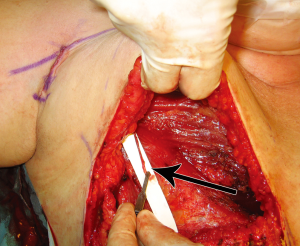
Venous anastomoses were performed with anastomotic devices that achieve fast anastomotic times: either ‘Anastoclip’ Vascular Closure Staples (VCS) micro-staple clips (AnastoClip Vessel Closure System, Le Maitre Vascular Inc, Sulzbach, Germany) or a microvascular anastomotic coupling device (Microvascular Anastomotic Coupling System, Synovis Micro Companies Alliance Inc, St Paul, Minnesota, USA), advanced anastomotic devices have been developed to aid arterial and venous anastomosis, with the use of staples and the ‘anastomotic ring coupler’ being the more widely discussed modern techniques. A recent article by Camara et al. described the use of anastomotic couplers for use in free deep inferior epigastric artery perforator (DIEP) flap surgery (25), which demonstrated the utility of the anastomotic coupler in 12 free flaps, and we have now utilized these devices in over 1,000 free flaps. Our experience is described in a subsequent subsection below.
In this series, a total of 564 DIEP flap breast reconstructions were performed in 501 patients, with 438 unilateral and 63 bilateral reconstructions. Of these, 273 breast reconstructions were performed in which only a single venous outflow route was implemented, and 291 cases had two veins used primarily for venous outflow. The DIEV was the primary source of venous drainage in all cases, and for secondary venous drainage, the SIEV was used most commonly (92.1%), followed by a second DIEV (7.9%). In the vast majority of cases where an SIEV was used, the cephalic vein was harvested as the recipient vein for these anastomoses (82.8% overall). There were no differences in outcomes when each of these venous outflow routes were compared for venous congestion (0 cases in either group). Of note, the use of a secondary vein did not result in any increase in operative time (385 vs. 383 minutes, P=0.57).
Of the 273 flaps in which a single vein was used, 7 flaps demonstrated venous congestion on clinical examination postoperatively. Of the other 291 flaps, which received an additional vein during initial breast reconstruction, no flaps demonstrated any signs of venous congestion. This decrease in the rate of venous congestion with the use of 2 veins was statistically significant, P=0.006. Of the 7 congested flaps, 5 were due to venous thrombosis and 2 were due to relative venous congestion with no pedicle compromise. All cases of venous congestion were taken back to theatre for re-exploration, and all cases of pedicle compromise were taken back to theatre for re-exploration, with the ultimate cause for compromise identified in theatre. Other complications were statistically similar between the groups, including complete flap failures (due to either arterial or venous thrombosis), partial flap losses, arterial or venous complications and overall take-backs.
Notably, while there were 5 cases of venous thrombosis in each group, all cases in which venous thrombosis did occur in the one vein group resulted in global venous congestion identified on examination (5/5=100%), however in the two vein group, venous thrombosis in a single vein (identified with the implantable Doppler probe) did not result in any clinical suggestion of venous congestion in any cases (0/5=0). There were no cases in which venous thrombosis occurred in both veins in the two vein group. In the two vein group, venous thrombosis was identified with the implantable Doppler probe and findings at theatre, rather than the clinical manifestations of venous failure. Of the cases of venous thrombosis, one case of venous thrombosis resulted in complete flap failure in the one vein group (1/5=20%), while no cases resulted in complete flap failure in the two vein group (0/5=0%). All other cases of complete failure flap were due to arterial thrombosis. This study demonstrated that by prospectively embarking on a second venous anastomosis, the venous drainage of a free flap can be significantly improved, reducing the incidence of venous congestion. The study also demonstrated that this can be readily achieved, without any demonstrable increase in operative times if planned effectively. In our series of over 500 DIEP flaps, we reduced our venous congestion rate to zero if a secondary vein was performed.
Use of the cephalic vein for secondary venous drainage
Given the clinically significant benefit in using a secondary route for venous drainage in the DIEP flap, the cephalic vein lends itself to a readily accessible option with minimal donor site morbidity (24). In preparing the patient, the arm is placed on an arm-board that enables abduction and draped appropriately. The delto-pectoral groove is marked and this line is extended out onto the arm as the cranial-most limit of the incision. An anterior axillary skin crease is identified where it meets the delto-pectoral groove (at the drawn line), and a line is drawn caudally along this crease for two to four centimeters to mark the line of incision. The subcutis is incised until the muscular fascia is reached. The dissection is continued in the cranial part of the wound until the fat pad between the deltoid and pectoralis major is identified. The fascia covering the fat is opened and the cephalic vein should be easily exposed (Figure 6).
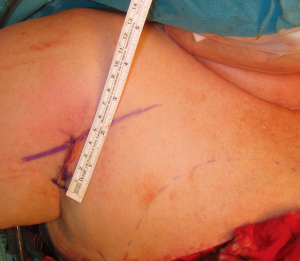
Blunt dissection is then performed with a finger to expose the path of the vein, following the cephalic vein laterally along the arm. The vein is then harvested medially, ligating and dividing small branches, usually followed until it dives towards the subclavian vein. The dissection is then continued laterally, isolating the vein as far as can be reached with long scissors and long DeBakey forceps. If a longer vein is needed, this can easily be achieved by following the vein through small incisions distally on the arm. A headlamp or a lighted retractor can be used to aid vision. The vein is then clamped with vascular clips and divided. A subcutaneous tunnel from the flap recipient site (chest wall) to the delto-pectoral groove is created, and the vein delivered through it (Figure 7). We have observed a good size match between the SIEV and cephalic vein in all of our cases, and have had no harvest-site morbidity, with a scar less than 4cm long in all cases (Figure 8).
With the ease and speed of performing cephalic vein harvest as described, we perform a secondary venous anastomosis prospectively, before venous complications arise, and have had no venous compromise in any of our flaps using this technique. Our choice is based on the assessment of the dominance of the SIEV for venous drainage: if the diameter is large on preoperative imaging or intraoperatively, or if there is a distended or high pressure SIEV upon venous pressure measurements after cannulation, we will prospectively perform a second anastomosis.
Venous anastomoses
Advanced anastomotic devices have been developed to aid venous anastomosis, given the importance of venous drainage to flap survival. While suturing has been the traditional mainstay, staples and the ‘anastomotic ring coupler’ have been the more widely discussed modern techniques. Our cohort study comprised 1,000 consecutive patients undergoing a range of reconstructive procedures, recruited through a single institution (26). This comprised breast reconstructive cases (600 cases), extremity reconstruction (150 cases) and head and neck cases (250 cases). Three modes of vascular anastomosis were performed: standard sutures, the ‘Anastoclip’ Vascular Closure Staples (VCS) micro-staple clips (AnastoClip Vessel Closure System, Le Maitre Vascular Inc, Sulzbach, Germany) and a microvascular anastomotic coupling device (Microvascular Anastomotic Coupling System, Synovis Micro Companies Alliance Inc, St Paul, MN, USA). Our preliminary use of these anastomotic procedures has been described (27).
The devices were applied intraoperatively by the primary surgeon in each case, with decision to use the particular device at the discretion of the surgeon. There were no particular intraoperative criteria for selection of a particular anastomotic technique. For the ring coupler, vessel wall eversion was achieved with the device itself, while for stapled anastomoses eversion was achieved with a combination of stay sutures and everting forceps in order to achieve ideal intima-to-intima apposition. Two main outcome parameters were assessed: anastomotic time and anastomotic failure. Anastomotic times were recorded by the scout nurse in each case, and anastomotic failure was confirmed in theatre at revision surgery. Data were analysed statistically with the Fisher exact test, with a P-value of less than 0.05 considered as having statistical significance. In 1,000 reconstructive cases, 2,500 vascular anastomoses were performed, of which 1,400 comprised the use of either of the two adjunctive anastomotic devices. These devices were thus used in 80% of all venous anastomoses and 10% of all arterial anastomoses, with the anastomotic ring coupler used in 1,000 cases and staples in 400 cases. In cases of sutured anastomoses, mean anastomotic time was 22 minutes, compared to 15 minutes with staples and 4 minutes for the ring coupler, a significant reduction in anastomotic times with the use of these devices (P<0.01). In terms of anastomotic failures, there were 90 failures overall, of which 12 were arterial failures and 78 were venous failures. Of these, all arterial failures were sutured (no stapled or coupled arterial anastomoses failed), and of the venous failures, 29 were sutured, 20 were stapled and 29 were coupled (P>0.05). In 1,000 free flaps in which we used the anastomotic coupler, the mean reduction in time with the use of a ring coupled anastomosis was 15 minutes as compared to sutured anastomosis (P<0.001). The surgical anastomosis itself is just one of several aspects of microvascular surgery that have reaped the reward of technological advances in the field: The ability to preoperatively select the optimal vessels of choice for inclusion in the vascular pedicle has been shown to improve a range of operative outcomes, with techniques that have now been established comprising color duplex ultrasound (28), computed tomographic angiography (29), and magnetic resonance angiography (30); developing and future techniques include the use of ‘virtual’ image-guided stereotactic navigation pre- and intra-operatively (31); intraoperative mapping of vasculature have been shown to accurately map the vascular territories of selected vessels, enabling improved flap design and harvest (32); and vascular monitoring postoperatively has also been revolutionized with the use of pedicle monitoring techniques such as the implantable Doppler probe (see subsection below), which has been shown to rapidly identify vascular complications (such as thrombosis, compression or kinking) and potentiate rapid return to theatre and flap salvage (33).
Postoperative venous monitoring
The Cook-Swartz implantable Doppler probe has become increasingly recognized as a useful tool for the postoperative monitoring of free flaps (33-39). With the potential for either flap salvage or flap loss, prompt and effective postoperative evaluation of flap viability is essential, and may potentiate early intervention. While the no-reflow phenomenon will still mean that flaps are lost regardless of the experience of the microsurgeon, optimal methods for post-operative monitoring of free flaps have become increasingly sought.
A broad range of different technologies have been discussed for post-operative monitoring, however there is little evidence for any single technique. This is reflected in the wide variety of techniques currently used in this role: clinical monitoring alone, pulse oximetry, near infra-red spectroscopy (NIRS), perfusion photoplethysmography, surface temperature measurement, fluorometry, microdialysis, ultrasound, the hand-held Doppler probe, implanted (Cook-Swartz) Doppler probes, laser Doppler flowmetry, impedance plethysmography, confocal microscopy, nuclear medicine, subcutaneous pH measurement, hydrogen clearance, externalisation of part of a buried flap and white light spectrometry.
We have used three established monitoring techniques in a recent and comparable cohort of patients. Three different techniques were utilised as the primary mode for postoperative monitoring: the implantable Cook-Swartz Doppler probe, microdialysis and clinical monitoring alone, all techniques which have been previously shown to accurately predict the onset and existence of flap compromise (Figures 9,10,11). Clinical monitoring alone, the implantable Doppler probe and microdialysis all showed statistically similar rates of flap salvage, however there was a statistically significant increase in false positive alarms causing needless take-backs to theatre in the microdialysis and implantable Doppler arms, finding no technique superior to clinical monitoring alone.
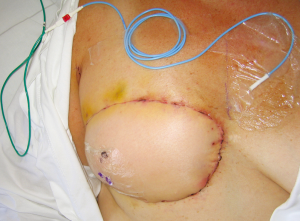
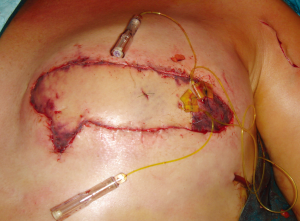
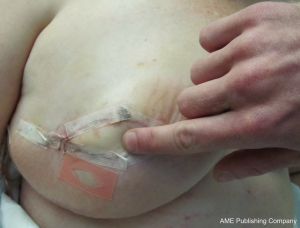
In using the implantable Doppler probe, our preference of the adjunct techniques available, the probe cuff is placed around the venous pedicle following successful venous anastomosis, and monitoring of the venous pedicle proceeds during flap insetting and throughout the early postoperative period (40). The Cook-Swartz implantable Doppler probe is either left unattached around the venous pedicle or is secured. In our combined experience with over 300 such applications of the Cook-Swartz implantable probe (Cook Medical®, Cook Ireland Ltd, Limerick, Ireland), we typically secure the silicone cuff with two small micro-clips as described previously (38). An alternative technique for attachment is to glue the cuff with the use of fibrin glue (41). These techniques require redundant silicone cuff for apposition, however we have encountered some vessels that are of sufficiently large diameter as to not provide enough cuff to employ these methods. The Cook–Swartz venous Doppler probe is adherent to a silicone cuff that is wrapped around the selected vessel. As per the manufacturer and literature, the probe is always used on the venous pedicle (as arterial compromise causes venous changes within minutes). The tension of the silicone cuff is highly important, as placement of a tight cuff may obstruct venous outflow, while placement of a loose cuff may result in loss of the Doppler signal or cause migration of the cuff. Metallic micro-clips are easily applicable for cuff attachment, achieved by opposing the redundant ends of the cuff before careful placement of the clips (Figure 12). In some cases vessel diameter or anatomy is such that after placement of the silicone cuff, there is no redundancy to the cuff ends and clips and/or glue are not able to be used. Placement without cuff attachment would increase the chance of false positive results, and thus we use two techniques in this setting to aid attachment. The first is to apply two interrupted sutures through the cuff ends to mimic the technique of micro-clips (Figure 13). The sutures can be tightened to the desired tension, and can be used in cases where the cuff ends are not in direct apposition. A second technique is to excise a segment of silicone cuff and either clip or suture the excised segment to the cuff ends, effectively elongating the cuff diameter.

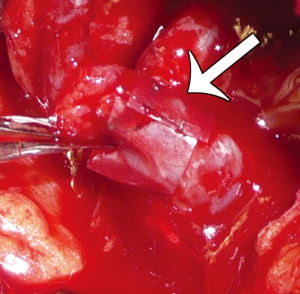
In our experience (over 300 cases) of using all four techniques (non-attachment, micro-clip fixation, suture fixation, silicone cuff elongation), no complications have been encountered as a consequence of the use of the implantable Doppler probe or as a consequence of the technique selected. In the first 300 of our cases, the breakdown of fixation techniques were as follows: 270 cases using micro-clips, 20 cases using suturing, 8 cases without fixation (early in our experience) and 2 cases using silicone cuff elongation. Of note, the technique of non-attachment was associated with an increased rate of false positive results, as migration away from the vessel being monitored was postulated to have occurred. The other three methods were not associated with any unique problems. In terms of outcome measures from the use of the implantable Doppler probe, there has been shown to be a strong trend toward improved salvage rates with the implantable Doppler probe compared with clinical monitoring (80% vs. 66%), and a meta-analysis has shown this to be statistically significant (P<0.01) (34). There was no statistical difference in false-positive rates in these series. In addition to the absence of complications in the application of the silicone cuff, we have similarly not encountered any complications as a result of probe removal. The probes are removed at the end of the monitoring period (typically at the end of the first postoperative week) by simply applying a minimal amount of traction on the wire where it exits the wound. This force detaches the wire from the silicone cuff, leaving the cuff in-situ. According to the manufacturers specifications, a force of only 50 grams is required to achieve detachment, and in cases where there is any resistance to traction, we cut the wire at the level of the skin and leave the internal length of wire in-situ. In all cases thus far, we have not encountered any complications during probe removal.
Through direct monitoring of the vascular pedicle, the implantable Doppler probe is an immediate reflection of impaired flow (42,43). The signal itself can be highly variable and can be associated with a substantial learning curve for interpretation. This is largely due to in-vivo changes in venous flow and offer a fascinating insight into venous anatomy and physiology. Despite an increased reporting of the use of this technique for monitoring flaps, there has not been a discussion of the bedside techniques available to aid interpretation of the audible Doppler signal (44).
It is important for medical and nursing staff to recognize that despite the probe always being applied to the venous pedicle, there are two main Doppler waveforms that are possible: venous and arterial. Venous flow is typically low-pitch and constant, resembling a humming sound or the sound of the ocean (Supplementary Video 1, 2). The arterial waveform can often predominate when the arterial pedicle is in close proximity to the vein, and is both louder than its venous counterpart and is pulsatile. Often a combination of each of these waveforms is heard, and in many cases there can be interchange between the predominant waveform during the postoperative period. The implantable Doppler signal can vary substantially in volume, pitch and quality throughout the postoperative period in any one patient. Significant changes often warrant re-exploration, but in equivocal cases, we have found several bedside tests can help to reassure the presence of pedicle flow. Such bedside tests can aid in both identification of the presence of an otherwise equivocal Doppler signal, and confirm appropriate flap flow after a Doppler signal change.
Respiratory variation
Respiratory variation in Doppler flow is particularly useful for flaps based on recipient vessels in the thorax, such as those for breast reconstruction. Respiratory action causes changes in intra-thoracic pressure which cause resultant increases and decreases in pedicle flow. These flow changes result in audible changes in volume and pitch, and confirm pedicle patency (Video 1).
Flap compression
Manual compression of a flap (whether a cutaneous or muscle flap) causes an artificial increase in venous outflow by emptying intra-flap veins, resulting in an audible increase in the volume and pitch of the Doppler signal (Video 2). This too confirms pedicle patency.
Elevation/dependency
Changes in Doppler signal occur with relative changes in the dependency of the recipient site, and this is particularly useful for lower limb recipient sites. By lowering and raising a limb, there are gravitational changes in venous outflow from a flap, with the resultant changes in Doppler signal confirming pedicle patency.
With a range of bedside clinical manoeuvres, reliability in interpreting the implantable Doppler signal can be improved, with the potential for a decrease in needless take-backs to theatre or missed pedicle compromise.
Venous anomalies and clinical cases
Dual SIEV trunks
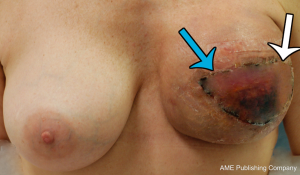
While the SIEV is classically considered to drain via a single trunk, two SIEVs draining into separate venous trunks was identified (45). While venous anatomy is known to be variable, this variant of normal anatomy had not been described previously, with clinical implications clearly warranting a review. Preoperative computed tomographic angiogram (CTA) to assess the vasculature of the anterior abdominal wall for flap planning was undertaken, and as shown in Figure 14, there was found to be a paucity of DIEA perforators, and rather dominant SIEAs bilaterally were identified. Large SIEVs were also seen adjacent to the SIEAs, which were considered suitable for donor venous drainage. The differentiation between the superficial arterial and venous systems was based upon careful three-dimensional, multi-planar analysis of the CTA, rather than a single image (as seen in Figure 14), tracing the vessels to their origins and destinations. As was our routine practise (and as described in the broader literature to date), the scan was carefully analysed in terms of arterial vasculature, while the venous anatomy was analysed only in terms of its presence and location. The branching pattern of the SIEV was not primarily considered in the process of flap planning. As such, the patient underwent an abdominal wall flap based on the right SIEA. The right hemiabdominal flap (to the midline) was raised on the right SIEA and SIEV. The SIEA (2.2 mm at its origin) and SIEV (2.5 mm) were anastomosed to the internal mammary artery and vein, with relatively good size match (2.9 and 3.0 mm respectively). The anastomoses were both sutured with interrupted nylon sutures, and there was good pedicle flow upon removal of the clamps.
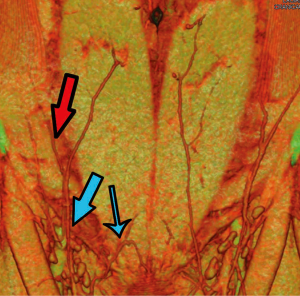
While perfusion was good throughout the length of the operation, relative venous congestion to half the flap was noted progressively throughout the early postoperative period. Of particular note was the clear demarcation of the congestion to half the flap only (Figure 15). The lack of global venous congestion highlighted that this was not a pedicle problem, but rather a territorial issue related to relative venous congestion. Rather than warranting immediate exploration, this suggested an expectant approach. While consideration of re-exploration was certainly given, we reviewed the patient’s preoperative CTA in order to explore any potential reasons for the area of venous compromise. Retrospective review of her CTA highlighted an interesting feature of her SIEV - there were two separate SIEV trunks on the right side, with only one (the lateral trunk) used to drain the flap (Figure 14). While clear that an additional venous anastomosis of the medial trunk is what would have been required in this case, this trunk was not prophylactically dissected for any substantial length, and thus was not a clinical option. This understanding of the cause of the congestion contributed to the decision for expectant management. Over the course of the postoperative period, the congestion gradually improved and ultimately a small area of fat necrosis was treated conservatively, with no reoperation performed.
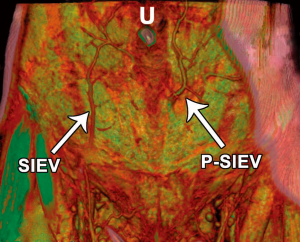
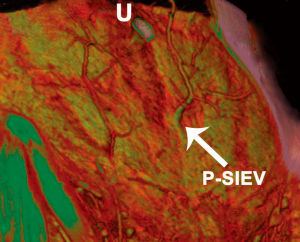
The ‘Perforating’ SIEV
With the use of preoperative CTA, a unique anomaly has been identified in which there is no superficial inferior epigastric vein (SIEV) entering the abdominal wall integument below the inguinal ligament, and instead arises from the DIEA itself and perforates the rectus abdominis muscle as a musculocutaneous perforator at a more proximal origin (46). This anomalous ‘perforating’ SIEV is a new anatomical variant detected with preoperative CTA. With an increasing detection of anatomical variation, the benefits of preoperative imaging before abdominal wall free flaps are further highlighted. A clinical study of 145 patients undergoing preoperative CTA for consecutive DIEA perforator flaps for breast reconstruction was undertaken. Scans were reviewed for the incidence of the ‘perforating’ SIEV, with anatomical features recorded. In five cases (3.4% of overall cases), the perforating SIEV variant was identified. In all cases there was no caudal SIEV originating from an inguinal vein (superficial femoral vein, saphenous bulb or long saphenous vein). Instead, a large SIEV originated from the DIEA and traversed the rectus abdominis muscle as a very large (>3 mm) musculocutaneous perforator. In all 5 cases, this originated from the medial branch of the DIEA as a medial row perforator, and perforated the rectus sheath approximately midway between the umbilicus and the arcuate line. In all cases, this perforator (combined artery and vein) was the largest perforator for the entire abdominal wall. Upon emerging from the anterior rectus sheath, the SIEV traversed the cutaneous tissues cranially (Figures 16,17).
Macrovascular arterio-venous shunts
We have been able to identify large shunts between arterial perforators and the superficial venous system (47). While we have until now highlighted the venous ‘half’ of the abdominal wall circulation, in which circulation from the heart, through arteries and arterioles to capillaries, and returning through venules and veins is well established. Alternative bypass networks are known to occur however, with ‘arteriovenous anastomoses’ (AVAs) well described anatomical structures which appear to be common throughout the circulatory system (48) with a few known exceptions. They are most easily defined as normally occurring, pre-capillary communications between the arterial and venous sides of the circulation, with most studies on this topic demonstrating their pre-capillary positioning. The term ‘shunt’ has been used interchangeably with AVA, as the function of these structures is to allow a bypass circuit around capillary beds. All of the literature on these structures have described AVAs of a usual calibre of 0.05-0.15 mm in diameter (48) with the largest described AVA being 0.5 mm (49), and all intimately associated with capillary beds.
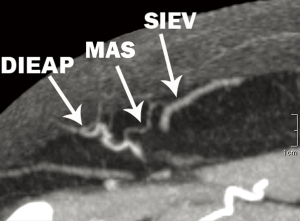
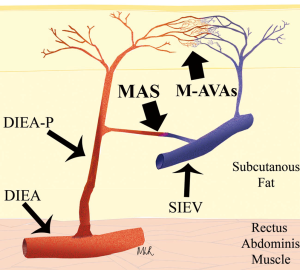
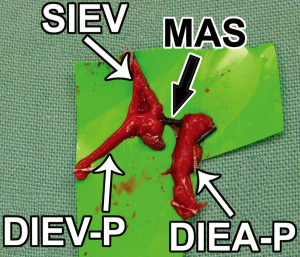
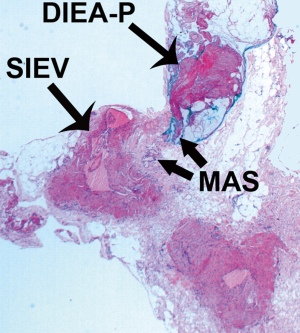
While all previous studies on the topic have utilized techniques such as histology, and pharmacological and/or physiological manipulation, the use of advanced imaging technologies such as computed tomographic angiography (CTA) has provided a new modality for investigating increasingly small calibre vessels. We have been able to identify radiological evidence of such as vessel: the macrovascular arteriovenous shunt (MAS). As shown in Figure 18 a, 1mm communication is clearly identifiable between a DIEA perforator and the SIEV, with a schematic of this anatomy shown in Figure 19. We have further evaluated the anatomical features of these shunts clinically and histologically (50). In Figure 20, the structure is seen in-vivo at high-power magnification, demonstrating the MAS communication between a DIEA perforator (DIEA-P) and the SIEV (Figure 20). Upon excision of this structure, the anatomy has been displayed ex-vivo (Figure 21). Figure 22 demonstrates the histology of the MAS, with ‘arterial’ features on the arterial side of the shunt and ‘venous’ features on the venous side (Figure 22).

These MAS provide vascular shunting prior to capillary filling and have potentially profound clinical implications and therapeutic possibilities in a range of medical and surgical conditions. An understanding of the autonomic supply to these shunts and potential role for pharmaceutical manipulation in free flap surgery offer an important field of future research.
Discussion
The implications of inadequate venous drainage of DIEA perforator flaps are great, with the potential for partial or complete flap loss (1). However, the cause for venous compromise in individual cases is difficult to either predict preoperatively or select intraoperatively. By identifying the features of draining veins that optimize venous drainage, selection of those perforating veins with optimal anatomy or selection of using the superficial venous system can be made preoperatively for inclusion in the flap.
Previous anatomical studies have indeed sought to achieve this, using cadaveric or excisional specimen models to assess the direction of venous flow past valves (10,17-20). A limitation of these techniques includes the ex-vivo changes in vascular anatomy that may influence findings. In addition, early studies focused their observations on the implications for pedicled abdominal wall flaps, such as pedicled transverse rectus abdominis myocutaneous (TRAM) flaps, with limited application to the DIEP flap, in which only 1-3 perforators are included in the venous drainage of the flap. Despite these limitations, these studies were able to demonstrate that the direction of venous flow is preferentially from the superficial venous system to the deep venous system, through a series of perforating veins. This direction of flow, and the dominance of the superficial venous system in the drainage of the abdominal wall, is a finding confirmed in the current study.
As a DIEP flap is not drained by the dominant drainage system, and relies upon 1-3 perforating veins to drain an entire flap, factors relating to the venous anatomy have been postulated in the pathogenesis of venous problems in the raising of a DIEP flap. In these previous studies, the size of venous perforators has been highlighted as a significant contributing factor. Cases have been identified where there have been no suitable perforating veins in an entire hemiabdominal wall, with the proposition that venous compromise is “probably due to the sacrifice of a critical number of venous perforators” (17). This hypothesis, that the calibre of perforating veins is the limiting factor to venous drainage, has been shown experimentally to be true (51,52), and has been echoed more recently in a clinical study of the DIEA perforator flap (11).
In addition to the size of perforators, an additional factor implicated in the causality of venous compromise has been the degree of midline crossover by the SIEV (10,21). While a single perforator may be of sufficient calibre to drain the flap, its communication with veins of each side of the abdominal wall is also essential. Blondeel et al. found a lack of midline crossover by the SIEV in 36% of specimens, while Schaverien et al. identified a case without midline crossover. Both these studies postulated this midline crossover as a cause of venous problems. In our series, there was no midline crossover in 1/16 cadaveric specimens and in 14/100 clinical cases (13% of cases overall).
In addition to these factors, there may be a further anatomical factor in the pathogenesis of venous compromise in DIEP flaps. The communication between the perforating vein and the SIEV is not uniform, and frequently this communication is of substantially smaller calibre than either the SIEV or the perforating veins themselves. These communications, described as oscillating veins between the adjacent venous territories (20), may be the limiting factor to venous flow in many cases. With the use of CTA in the current study, the presence and calibre of these communications are readily identifiable and can be identified preoperatively. The use of identification of the presence and calibre of this communication in selecting the optimal perforators for inclusion in the vascular supply of the flap has been described, and shown to be a useful tool for aiding perforator selection and for maximizing venous outflow (53). Throughout our previous anatomical and clinical studies, we have identified the anatomical basis for this, and confirmed the utility of CTA in evaluating this anatomy. With this anatomy essential to flap survival in DIEP flap surgery, and continuing evolution in our understanding of this anatomy, future research will no doubt further improve our appreciation of this intricate anatomy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Rozen WM, Pan WR, Le Roux CM, et al. The venous anatomy of the anterior abdominal wall: an anatomical and clinical study. Plast Reconstr Surg 2009;124:848-53. [Crossref] [PubMed]
- Enajat M, Rozen WM, Whitaker IS, et al. A single center comparison of one versus two venous anastomoses in 564 consecutive DIEP flaps: investigating the effect on venous congestion and flap survival. Microsurgery 2010;30:185-91. [Crossref] [PubMed]
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Blondeel N, Vanderstraeten GG, Monstrey SJ, et al. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br J Plast Surg 1997;50:322-30. [Crossref] [PubMed]
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Nahabedian MY, Momen B, Galdino G, et al. Breast Reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg 2002;110:466-75. [Crossref] [PubMed]
- Boyd JB, Taylor GI, Corlett RJ. The vascular territories of the superior epigastric and deep inferior epigastric systems. Plast Reconstr Surg 1984;73:1-16. [Crossref] [PubMed]
- Taylor GI, Watterson PA, Zelt RG. The vascular anatomy of the anterior abdominal wall: the basis of flap design. Perspect Plast Surg 1991;5:1-30.
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [Crossref] [PubMed]
- Blondeel PN, Arnstein M, Verstraete K, et al. Venous congestion and blood flow in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg 2000;106:1295-9. [Crossref] [PubMed]
- Wechselberger G, Schoeller T, Bauer T, et al. Venous superdrainage in deep inferior epigastric perforator flap breast reconstruction. Plast Reconstr Surg 2001;108:162-6. [Crossref] [PubMed]
- Marck KW, van der Biezen JJ, Dol JA. Internal mammary artery and vein supercharge in TRAM flap breast reconstruction. Microsurgery 1996;17:371-4. [Crossref] [PubMed]
- Tutor EG, Auba C, Benito A, et al. Easy venous superdrainage in DIEP flap breast reconstruction through the intercostal branch. J Reconstr Microsurg 2002;18:595-8. [Crossref] [PubMed]
- Niranjan NS, Khandwala AR, Mackenzie DM. Venous augmentation of the free TRAM flap. Br J Plast Surg 2001;54:335-7. [Crossref] [PubMed]
- Villafane O, Gahankari D, Webster M. Superficial inferior epigastric vein (SIEV): ‘lifeboat’ for DIEP/TRAM flaps. Br J Plast Surg 1999;52:599. [Crossref] [PubMed]
- Lundberg J, Mark H. Avoidance of complications after the use of deep inferior epigastric perforator flaps for reconstruction of the breast. Scand J Plast Reconstr Surg Hand Surg 2006;40:79-81. [Crossref] [PubMed]
- Carramenha e Costa MA, Carriquiry C, Vasconez LO, et al. An anatomic study of the venous drainage of the transverse rectus abdominis musculocutaneous flap. Plast Reconstr Surg 1987;79:208-17. [Crossref] [PubMed]
- Moon HK, Taylor GI. The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg 1988;82:815-32. [Crossref] [PubMed]
- Taylor GI. In discussion of: Carramenha e Costa MA, Carriquiry C, Vasconez LO, Grotting JC, Herrera RH, Windle BH. An anatomic study of the venous drainage of the transverse rectus abdominis musculocutaneous flap. Plast Reconstr Surg 1987;79:208-17. Plast Reconstr Surg 1987;79:214-7.
- Taylor GI, Caddy CM, Watterson PA, et al. The venous territories (venosomes) of the human body: experimental study and clinical implications. Plast Reconstr Surg 1990;86:185-213. [Crossref] [PubMed]
- Schaverien M, Saint-Cyr M, Arbique G, et al. Arterial and venous anatomies of the deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg 2008;121:1909-19. [Crossref] [PubMed]
- Taylor GI. Angiosomes of the Human Body [MD]. Parkville: The University of Melbourne; 1990.
- Rees MJ, Taylor GI. A simplified lead oxide cadaver injection technique. Plast Reconstr Surg 1986;77:141-5. [Crossref] [PubMed]
- Audolfsson T, Rozen WM, Wagstaff MJ, et al. A reliable and aesthetic technique for cephalic vein harvest in DIEP flap surgery. J Reconstr Microsurg 2009;25:319-21. [Crossref] [PubMed]
- Camara O, Herrmann J, Egbe A, et al. Venous coupler for free-flap anastomosis. Anticancer Res 2009;29:2827-30. [PubMed]
- Rozen WM, Whitaker IS, Acosta R. Venous coupler for free-flap anastomosis: outcomes of 1,000 cases. Anticancer Res 2010;30:1293-4. [PubMed]
- Zeebregts C, Acosta R, Bölander L, et al. Clinical experience with non-penetrating vascular clips in free-flap reconstructions. Br J Plast Surg 2002;55:105-10. [Crossref] [PubMed]
- Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of Computed Tomographic Angiography and Doppler ultrasound. Plast Reconstr Surg 2008;121:9-16. [Crossref] [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. Planning and optimizing DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconstr Aesthet Surg 2010;63:289-97. [Crossref] [PubMed]
- Rozen WM, Stella DL, Bowden J, et al. Advances in the preoperative planning of DIEA perforator flaps: magnetic resonance angiography. Microsurgery 2009;29:119-23. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Stella DL, et al. Stereotactic image-guided navigation in the preoperative imaging of perforators for DIEP flap breast reconstruction. Microsurgery 2008;28:417-23. [Crossref] [PubMed]
- Holm C, Mayr M, Höfter E, et al. Interindividual variability of the SIEA angiosome: effects on operative strategies in breast reconstruction. Plast Reconstr Surg 2008;122:1612-20. [Crossref] [PubMed]
- Smit JM, Whitaker IS, Liss AG, et al. Post operative monitoring of microvascular breast reconstructions using the implantable Cook-Swartz doppler system: A study of 145 probes & technical discussion. J Plast Reconstr Aesthet Surg 2009;62:1286-92. [Crossref] [PubMed]
- Rozen WM, Chubb D, Whitaker IS, et al. The efficacy of postoperative monitoring: A single surgeon comparison of clinical monitoring and the implantable Doppler probe in 547 consecutive free flaps. Microsurgery 2010;30:105-10. [Crossref] [PubMed]
- Whitaker IS, Karoo ROS, Oliver DW, et al. Current techniques in the post-operative monitoring of microvascular free-tissue transfers. Eur J Plast Surg 2005;27:315-21. [Crossref]
- Whitaker IS, Oliver DW, Ganchi PA. Postoperative monitoring of microvascular tissue transfers: current practice in the United kingdom and Ireland. Plast Reconstr Surg 2003;111:2118-9. [PubMed]
- Whitaker IS, Rozen WM, Chubb D, et al. Postoperative monitoring of free flaps in autologous breast reconstruction: a multicenter comparison of 398 flaps using clinical monitoring, microdialysis, and the implantable Doppler probe. J Reconstr Microsurg 2010;26:409-16. [Crossref] [PubMed]
- Whitaker IS, Smit JM, Acosta R. A simple method of implantable Doppler cuff attachment: experience in 150 DIEP breast reconstructions. J Plast Reconstr Aesthet Surg 2008;61:1251-2. [Crossref] [PubMed]
- Rozen WM, Whitaker IS, Wagstaff MJD, et al. Buried free flaps for breast reconstruction: a new technique using the Cook-Swartz implantable Doppler probe for postoperative monitoring. Plast Reconstr Surg 2010;125:171e-2e. [Crossref] [PubMed]
- Rozen WM, Ang GG, McDonald AH, et al. Sutured attachment of the implantable Doppler probe cuff for large or complex pedicles in free tissue transfer. J Reconstr Microsurg 2011;27:99-102. [Crossref] [PubMed]
- Bill TJ, Foresman PA, Rodeheaver GT, et al. Fibrin sealant: a novel method of fixation for an implantable ultrasonic microDoppler probe. J Reconstr Microsurg 2001;17:257-62. [Crossref] [PubMed]
- Swartz WM, Izquierdo R, Miller MJ. Implantable venous Doppler microvascular monitoring: laboratory investigation and clinical results. Plast Reconstr Surg 1994;93:152-63. [Crossref] [PubMed]
- Swartz WM, Jones NF, Cherup L, et al. Direct monitoring of microvascular anastomoses with the 20-MHz ultrasonic Doppler probe: an experimental and clinical study. Plast Reconstr Surg 1988;81:149-61. [Crossref] [PubMed]
- Rozen WM, Ang GG, Acosta R, et al. Bedside manoeuvres and waveform changes in the interpretation of the implantable Doppler probe signal for free-flap monitoring. Microsurgery 2010;30:670-1. [Crossref] [PubMed]
- Rozen WM, Chubb D, Whitaker IS, et al. The importance of the superficial venous anatomy of the abdominal wall in planning a superficial inferior epigastric artery (SIEA) flap: case report and clinical study. Microsurgery 2011;31:454-7. [Crossref] [PubMed]
- Rozen WM, Grinsell D, Ashton MW. The perforating superficial inferior epigastric vein: a new anatomical variant detected with computed tomographic angiography. Plast Reconstr Surg 2010;125:119e-20e. [Crossref] [PubMed]
- Rozen WM, Chubb D, Ashton MW, et al. Macrovascular arteriovenous shunts (MAS): a newly identified structure in the abdominal wall with implications for thermoregulation and free tissue transfer. J Plast Reconstr Aesthet Surg 2010;63:1294-9. [Crossref] [PubMed]
- SHERMAN JL Jr. Normal Arteriovenous Anastomoses. Medicine (Baltimore) 1963;42:247-67. [Crossref] [PubMed]
- Tobin CE, Zariquiey MO. Arterio-venous shunts in the human lung. Proc Soc Exp Bio Med 1950;75.
- Rozen WM, Chubb D, Ashton MW, et al. Images in plastic surgery: the anatomy of macrovascular arteriovenous shunts and implications for abdominal wall free flaps. Ann Plast Surg 2011;67:99-100. [Crossref] [PubMed]
- Chow SP, Chen DZ, Gu YD. The significance of venous drainage in free flap transfer. Plast Reconstr Surg 1993;91:713-5. [Crossref] [PubMed]
- Miles DA, Crosby NL, Clapson JB. The role of the venous system in the abdominal flap of the rat. Plast Reconstr Surg 1997;99:2030-3. [Crossref] [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. Planning and optimizing DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconstr Aesthet Surg 2010;63:289-97. [Crossref] [PubMed]


