Radiotherapy and breast reconstruction: oncology, cosmesis and complications
Abstract
Breast reconstruction plays a highly important role in the management of patients with breast cancer, from a psycho-social and sexual stand-point. Given that immediate breast reconstruction does not impair the oncologic safety of breast cancer management, with no increase in local recurrence rates, and no delays in the initiation of adjuvant chemotherapy or radiotherapy, the need to balance cosmesis in reconstruction with the oncologic needs of breast cancer patients is no more evident than in the discussion of radiotherapy. Radiotherapy is essential adjuvant therapy in the treatment of breast cancer, with the use of adjuvant radiotherapy widely shown to reduce local recurrence after both partial and total mastectomy and shown to prolong both disease-free and overall survival in patients with nodal disease. In the setting of breast reconstruction, the effects of radiotherapy are potentially two-fold, with consideration required of the impact of breast reconstruction on the administration of and the initiation of radiotherapy, as well as the effects of radiotherapy on operative complications and cosmetic outcome following immediate breast reconstruction. The current editorial piece aims to analyze this balance, contrasting both autologous and implant-based reconstruction. The literature is still evolving as to the relative role of autologous vs. alloplastic reconstruction in the setting of radiotherapy, and the more recent introduction of acellular dermal matrix and other compounds further complicate the evidence. Fat grafting and evolving techniques in breast reconstruction will herald new discussions on this front.
Key words
Implant; reconstructive surgery; radiation; adjuvant therapy; breast reconstruction
The need to balance cosmesis in reconstruction with the oncologic needs of breast cancer patients is no more evident than in the discussion of radiotherapy (1). Radiotherapy is essential adjuvant therapy in the treatment of breast cancer, with the use of adjuvant radiotherapy widely shown to reduce local recurrence after both partial and total mastectomy and shown to prolong both disease-free and overall survival in patients with nodal disease (1-6). In the setting of breast reconstruction, the effects of radiotherapy are potentially two-fold, with consideration required of the impact of breast reconstruction on the administration of and the initiation of radiotherapy, as well as the effects of radiotherapy on operative complications and cosmetic outcome following immediate breast reconstruction. The current editorial piece aims to analyze this balance, contrasting both autologous and implant-based reconstruction.
Oncologic issues
The impact of breast reconstruction on delaying the administration of radiotherapy has been explored in relatively few recent studies (7-11). This is surprising given the importance of the issue, with several significant studies demonstrating poorer oncologic outcomes with delays in radiotherapy (12-15). In fact, those studies which have addressed the issue have all been relatively low in numbers and based at single institutions. Each of these studies showed no delay in the initiation of adjuvant radiotherapy in patients undergoing immediate breast reconstruction. Breast reconstruction may also impact the delivery of radiotherapy, by means of distorting the chest wall anatomy and thus altering the design of the radiotherapy fields. This is in the setting of radiation fields which include the chest wall, internal mammary lymph nodes, supraclavicular lymph nodes and the apex of the axilla. Distorting the anatomy with a reconstructive flap or implant may diminish the radiation administered to these regions, or more commonly, may dictate the need for a wider radiation field (16-18).
The mode of action of radiotherapy involves the use of ionizing radiation, delivered by external beam radiation, to the chest wall and/or the surrounding lymph nodes. It is the mechanism of this effect, via direct disruption of protein and DNA molecules and the formation of free radicals and electrons causing molecular damage, that dictates both positive and negative outcomes (15). While these effects are directly toxic to malignant cells, radiation also damages healthy tissue. Direct tissue cellular damage with chromosomal alteration, microvascular occlusion with ischemia and inhibition of fibroblast action, are all implicated as mechanisms in tissue damage (19-22), leading to progressive loss of endothelial cells in the walls of microvsculature and leading to characteristic blind ending capillaries and regional ischaemia. Structural changes to the skin include changes in epidermal and dermal keratinocytes and melanocytes, damage to skin appendages, skin thinning and fibrosis (19,21,22). These damaging tissue responses are associated with the increased incidence of operative complications, particularly those associated with healing.
Reconstructive outcomes
In the setting of implant reconstruction, adjuvant radiotherapy has been widely described as having an unacceptably high complication rate, particularly the complications of capsular contracture, and rupture of the implant envelope or fibrous capsule (23,24). This is particularly true for postoperative radiotherapy, but has been associated with preoperative radiotherapy as well. Where post-operative radiotherapy is predicted, such as those high-risk cancers that are large, multifocal or have lymph node involvement, implant reconstruction has been described widely as an ill-advised option. Many of the studies showing this were associated with older regimes and modes of administration of radiotherapy, and more recent techniques, such as helical tomographic radiotherapy, may improve outcomes in the setting of breast reconstruction (25).
While the same conclus ions for autologous reconstruction have certainly been less rigid, there has been no consensus in the literature. In fact, our experience suggests that there are indeed complications in autologous reconstruction from radiotherapy, and that the effects of radiotherapy on implants in the setting of skin-sparing mastectomies may be less than previously suggested. The differences between autologous and implant reconstruction in this setting may thus be more comparable than previously suggested (1,23,26-28). Figures 1, 2, 3 highlight the effects of radiotherapy on autologous tissues alone, and highlight that these effects are not solely related to the alloplastic implant (Figures 1, 2, 3).
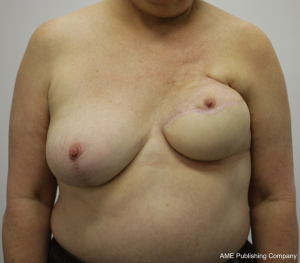
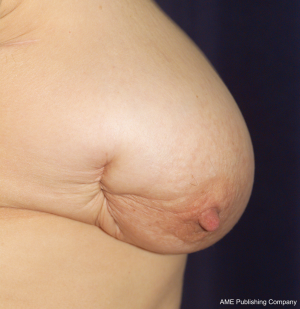
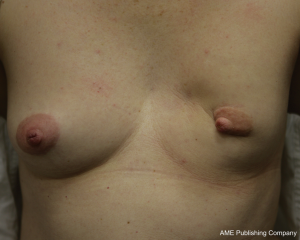
The effect of radiotherapy on operative outcome has been explored to a large extent, but not in any randomized control trials. For autologous reconstruction, there is conflicting data, with the timing of radiotherapy of importance. The complications that occur after autologous reconstruction in the previously irradiated chest are similar to those occurring in the setting of no radiation. However, given that the tissues have been afflicted with radiation damage, wound complications are more likely to be increased. Autologous reconstruction nevertheless, allows removal of some of the damaged tissue and the importation of donor healthy (non-irradiated) tissue.
The outcome of autologous reconstruction in the setting of previous (neoadjuvant) radiotherapy has been described in a large number of past studies (11,29-44), ranging from small, non-controlled studies, to large studies with over 100 cases that have been matched to a non-irradiated group. This diversity is echoed in their findings, with some of the larger studies demonstrating no significant difference in outcome and some showing significant increases in complication rates. The largest study was that of Williams et al. (1995), in which 118 patients with TRAM flap reconstruction after previous radiotherapy were compared to 572 patients without prior radiotherapy, with this study showing an increase in fat necrosis in patients with prior radiotherapy, but no increase in overall complications (35). Of the larger studies that assessed cosmetic outcome in the setting of previous radiotherapy, there were significantly poorer cosmetic scores (31,32). However, the overall incidence of these complications were not high, and as such autologous reconstruction is still considered safe after neoadjuvant radiotherapy. An additional consideration of preoperative radiotherapy is that it may reduce the incidence of loco-regional recurrence and increase disease free survival, thus reducing the incidence of local recurrence following reconstruction (1,45,46).
The outcomes following autologous reconstruction with subsequent adjuvant radiotherapy has been similarly explored widely, with variable results (34,41,42,47-55). Although most studies described extremely low flap loss rates, the incidence of tissue complications was generally greater than comparative groups, particularly that of fat necrosis. Several studies documented fat necrosis rates of greater than 20% (34,41,47,48). The largest study however, by Huang et al. (2006), did not demonstrate high complication rates, with a 0 flap loss and 8.5% incidence of fat necrosis, a figure comparable to those without adjuvant radiotherapy (51). Despite this, if radiotherapy is expected, delaying the reconstruction is the preferred mode of management because all too often the authors have witnessed the effect of post-reconstruction radiotherapy on well matched autologous reconstruction, resulting in fibrosis, volume loss and displacement and elevation of the nipple and areolar complex (Figure 1).
With the more widespread use of skin-sparing mastectomy (SSM) techniques, since the concept of preoperative plastic surgery planning together with SSM was first brought to the forefront by Toth et al. in 1991, an improvement in outcomes with implant reconstruction has developed (56,57). This involves the preservation of a native skin envelope with the removal of the breast, nipple-areolar complex, biopsy scars and skin overlying any superficial tumours, and the ideal SSM having a skin flap devoid of all breast tissue but having an adequate blood supply to prevent flap necrosis and delayed wound healing. It is believed that the preservation of the skin architecture and intact infra-mammary fold allows for immediate breast reconstruction, thereby reducing the number of reoperations and improving the cosmetic appearance of the breast, and diminish the need for tissue expansion and/ or remodelling in the setting of radiotherapy. In many past studies, the expander/implant option was considered a poor option in post-mastectomy reconstruction, suggesting that tissue expansion was associated with a significantly higher complication rate (38,58-60). However, the field of implantbased reconstruction has undergone constant change, including the advent of dual chambers, anatomic and cohesive variations, texture modifications, and ever-evolving proprietary manipulation (Figure 4). As a result, implantbased reconstruction data are difficult to standardize between studies, or over any prolonged period of time. Similarly, size of implant, initial volume, final volume, and rapidity of expansion are tailored by individual surgeons to meet patient goals and expectations and can never be fully standardized. The development of skin-sparing and, more recently, nipple-sparing techniques also adds a distinct element to this variability.
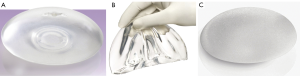
We were recently involved in a study exploring the outcome of breast implants following conservative mastectomy and SSM, examining the complication and reoperation rates in patients who underwent delayed versus immediate reconstruction, as well as patients who did and did not undergo radiation therapy (28). In several hundred patients, we found the overall complication rate of our implant-based reconstruction to be 15%, with a reoperation rate of 10%. This is lower than many of the previously described studies. Not only were we able to conclude that implant-based reconstruction can be associated with a low complication rate, even in the setting of radiotherapy, but that immediate reconstruction is also associated with a statistically significant lower reoperation rate. Previous studies have concluded that radiation therapy is associated with an unacceptably high rate of capsular contracture and rupture of the implant envelope or capsule (1,22,61), with a study by Spear and Onweyu in 2000 comprising 40 consecutive patients undergoing staged expander/ implant placement and radiotherapy, showing a capsular contracture rate of 21% in the irradiated group vs. 0% in the control group (62). Our findings did not echo these. While we found irradiated breasts having a statistically higher reoperation rate, overall complication rates were similar to non-irradiated breasts, and we postulate that with improvements in the targeting of radiotherapy in order to limit damage to surrounding tissue, improved surgical techniques, or better quality of implants, past conclusions may be overstated to current thinking.
In comparing implant and autologous reconstruction, the literature is varied, with some authors finding no difference between autologous and implant reconstruction, both overall and in the setting of radiotherapy, with Rosen and colleagues finding that the complication rates were similar between TRAM and tissue expander/implant reconstruction for breast reconstruction (63), and this has been echoed in other series (64-66). In light of these findings, the studies described above have varied in their conclusions. Several conclude that delayed reconstruction results in fewer complications and better outcomes, and others suggest that immediate reconstruction is safe and has no adverse consequences over delaying reconstruction. A further compromise, the ‘delayed-immediate’ reconstruction has also been postulated, in which a twostage approach comprises a tissue-expander in the first stage, and autologous reconstruction ensuing if radiation is subsequently not required (67). The group at greatest risk for requiring adjuvant radiotherapy, those with locally advanced or multifocal disease, larger tumors and/or nodal metastases, certainly warrant greater consideration of a delayed reconstruction. However, this group is not always easily determined preoperatively, as although preoperative ultrasound can predict nodal status, there is a low sensitivity for small macro-metastases and/or micrometastases (68). Similarly, both axillary node frozen section and imprint cytology have significant false-negative rates making intraoperative prediction also difficult, and thus it is only post-operatively that a complete management plan can be formulated (69,70). As such, a significant number of those not expected to require adjuvant radiotherapy will ultimately be found to require it, warranting consideration of planning a delayed reconstruction from the outset.
A range of techniques have been introduced to ‘protect’ implants from the deleterious effects of radiotherapy. While the addition of overlying autologous tissues is an established technique, particularly with the use of local perforator flaps but also more distant regional or free flaps, more recent techniques have also been introduced. Acellular dermal matrix as an implant cover can reduce infection and capsular contracture rates even in the setting of radiotherapy (71), however the evidence for this is not yet well established, with more studies certainly needed (72).
Timing
Essential to the use of either implant or autologous reconstruction is the timing of both radiotherapy and reconstruction. In some settings, there is a preference to immediate radiotherapy, but where the oncology of the tumour permits delay to administration of radiotherapy, some principles can improve the reconstructive outcome. In implant reconstruction, there is a substantial benefit to maximising tissue expansion prior to radiotherapy - by allowing an inserted tissue expander to reach full volume and preferably to swap to a definitive implant prior to radiotherapy, the deleterious effects of radiotherapy can be minimised, in terms of soft tissue contracture and tissue loss. This will clearly eliminate the need to expand irradiated tissues, an almost impossible feat.
Autologous tissue transfer is advantageous in the irradiated situation, as the transfer of any non-irradiated tissue (whether locoregional or free tissue transplantation) into an irradiated bed will ‘revascularise’ that tissue and reduce the deleterious effects of the radiotherapy in the region - fibrosis, contracture and wound breakdown. If autologous tissue alone is planned for reconstruction, use of a tissue expander to hold the soft tissue envelope out to stretch and reaching a desired volume, can maximise the amount of available regional tissue, and minimise the amount of tissue needing transfer. Irradiation while fully expanded, but prior to free tissue transfer, can maximise these benefits, while maintaining the importation of nonirradiated tissue in a transferred flap.
Modern techniques
A range of techniques have been introduced to ‘protect’ implants from the deleterious effects of radiotherapy. While the addition of overlying autologous tissues is an established technique, particularly with the use of local perforator flaps but also more distant regional or free flaps, more recent techniques have also been introduced. Acellular dermal matrix as an implant cover can reduce infection and capsular contracture rates even in the setting of radiotherapy (71), however the evidence for this is not yet well established, with more studies certainly needed (72).
Fat grafting is another evolving technique in breast reconstruction that will herald new discussions on this front. Fat grafting has been successfully used to augment the reconstructed breast in the setting of both autologous and implant reconstruction (73-77), as well as being successfully used in the setting of primary breast reconstruction by fat grafting alone (78). In the setting of radiotherapy, there is discussion in the literature that the importation of tissue that becomes well-vascularised through grafting, particularly adipose-derived stem cells, can ‘revascularise’ the irradiated bed and reduce radiotherapy-related complications (77,79). The use of fat grafting in the breast to achieve these ends has been described for both pre-radiotherapy and post radiotherapy scenarios with benefit (76,80). Phulin et al. (2009) used fat grafting in irradiated head and neck tissues, and found an improvement in the quality of skin radiation damage after fat injection (80). They postulated that clinical improvement could be induced by an increase in vascularization and a revitalization of interstitial tissues, through an enhancement of angiogenesis via the secretion of growth factor and extracellular matrices. The adipose tissue is a potent source of multipotent stem cells, such as mesenchymal stem cells, and their intrinsic ability to secrete growth factors, in particular, angiogenic and antiapoptotic factors has been widely described (77,79).
As such, the literature is still evolving as to the relative role of autologous vs. alloplastic reconstruction in the setting of radiotherapy, and the more recent introduction of acellular dermal matrix and other compounds further complicate the evidence. Fat grafting and evolving techniques in breast reconstruction will herald new discussions on this front. What is clear is that breast reconstruction plays a highly important role in the management of patients with breast cancer, from a psycho-social and sexual stand-point, and that immediate breast reconstruction does not impair the oncologic safety of breast cancer management, with no increase in local recurrence rates, and no delays in the initiation of adjuvant chemotherapy or radiotherapy. Both neoadjuvant and adjuvant radiotherapy can increase the incidence of post-operative complications, with greater effects in the setting of postoperative radiotherapy, and if adjuvant radiotherapy can be predicted, a delayed reconstruction should be considered. However, a comparison of implant reconstruction to autologous techniques is not clear-cut.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Rozen WM, Ashton MW, Taylor GI. Defining the role for autologous breast reconstruction post-mastectomy: the social and oncological implications. Clin Breast Cancer 2008;8:134-42.
- Chua B, Olivotto IA, Weir L, et al. Increased use of adjuvant regional radiotherapy for node-positive breast cancer in British Columbia. Breast J 2004;10:38-44.
- Whelan TJ, Julian J, Wright J, et al. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 2000;18:1220-29.
- Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 1994;12:447-53.
- Vallis KA, Tannock IF. Postoperative radiotherapy for breast cancer: growing evidence for an impact on survival. J Natl Cancer Inst 2004;96:88-9.
- Early Breast Cancer Trialists’ Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet 2000;355:1757-70.
- Gouy S, Rouzier R, Missana MC, et al. Immediate reconstruction after neoadjuvant chemotherapy: effect on adjuvant treatment starting and survival. Ann Surg Oncol 2005;12:161-6.
- Salhab M, Al Sarakbi W, Joseph A, et al. Skin-sparing mastectomy and immediate breast reconstruction: patient satisfaction and clinical outcome. Int J Clin Oncol 2006;11:51-4.
- Foster RD, Esserman LJ, Anthony JP, et al. Skin-sparing mastectomy and immediate breast reconstruction: a prospective cohort study for the treatment of advanced stages of breast carcinoma. Ann Surg Oncol 2002;9:462-6.
- Downes KJ, Glatt BS, Kanchwala SK, et al. Skin-sparing mastectomy and immediate reconstruction is an acceptable treatment option for patients with high-risk breast carcinoma. Cancer 2005;103:906-13.
- Hultman CS, Daiza S. Skin-sparing mastectomy flap complications after breast reconstruction: review of incidence, management, and outcome. Ann Plast Surg 2003;50:249-55.
- Hershman DL, Wang X, McBride R, et al. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys 2006;65:1353-60.
- Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol 2003;21:555-63.
- Jobsen JJ, Van Der Palen J, Ong F, et al. Timing of radiotherapy and survival benefit in breast cancer. Breast Cancer Res Treat 2006;99:289-94.
- Jugenburg M, Disa JJ, Pusic AL, et al. Impact of radiotherapy on breast reconstruction. Clin Plast Surg 2007;34:29-37.
- Buchholz TA, Kronowitz SJ, Kuerer HM. Immediate breast reconstruction after skin-sparing mastectomy for the treatment of advanced breast cancer: radiation oncology considerations. Ann Surg Oncol 2002;9:820-1.
- Schechter NR, Strom EA, Perkins GH, et al. Immediate breast reconstruction can impact postmastectomy irradiation. Am J Clin Oncol 2005;28:485-94.
- Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:76-82.
- Mansfield C. Effects of radiation therapy on wound healing after mastectomy. Clin Plast Surg 1979;6:19-26.
- Robinson DW. Surgical problems in the excision and repair of radiated tissue. Plast Reconstr Surg 1975;55:41-9.
- Rudolph R. Complications of surgery for radiotherapy skin damage. Plast Reconstr Surg. 1982;70(2):179-185.
- Rudolph R, Arganese T, Woodward M. The ultrastructure and etiology of chronic radiotherapy damage in human skin. Ann Plast Surg 1982;9:282-92.
- Contant CM, Van Geel AN, Van Der Holt B, et al. Morbidity of immediate breast reconstruction (IBR) after mastectomy by a subpectorally placed silicone prosthesis: the adverse effect of radiotherapy. Eur J Surg Oncol 2000;26:344-50.
- Taylor CW, Horgan K, Dodwell D. Oncological aspects of breast reconstruction. Breast 2005;14:118-30.
- Massabeau C, Fournier-Bidoz N, Wakil G, et al. Implant breast reconstruction followed by radiotherapy: Can helical tomotherapy become a standard irradiation treatment? Med Dosim 2012. [Epub ahead of print].
- Evans GR, Schusterman MA, Kroll SS, et al. Reconstruction and the radiated breast: is there a role for implants? Plast Reconstr Surg 1995;96:1111-5.
- Vandeweyer E, Deraemaecker R. Radiation therapy after immediate breast reconstruction with implants. Plast Reconstr Surg 2000;106:56-9.
- Hughes K, Brown C, Perez V, et al. The effect of radiotherapy on implant-based breast reconstruction in the setting of skin-sparing mastectomy: clinical series and review of complications. Anticancer Res 2012;32:553-7.
- Hartrampf CR Jr, Bennett GK. Autogenous tissue reconstruction in the mastectomy patient. A critical review of 300 patients. Ann Surg 1987;205:508-19.
- Jacobsen WM, Meland NB, Woods JE. Autologous breast reconstruction with use of transverse rectus abdominis musculocutaneous flap: Mayo clinic experience with 147 cases. Mayo Clin Proc 1994;69:635-40.
- Kroll SS, Coffey JAJ, Winn RJ, et al. A comparison of factors affecting aesthetic outcomes of TRAM flap breast reconstructions. Plast Reconstr Surg 1995;96:860-4.
- Kroll SS, Schusterman MA, Reece GP, et al. Breast reconstruction with myocutaneous flaps in previously irradiated patients. Plast Reconstr Surg 1994;93:460-9.
- Salmon RJ, Razaboni R, Soussaline M. The use of the latissimus dorsi musculocutaneous flap following recurrence of cancer in irradiated breasts. Br J Plast Surg 1988;41:41-4.
- Tran NV, Chang DW, Gupta A, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg 2001;108:78-82.
- Williams JK. Bostwick Jr, Bried JT, et al. TRAM flap breast reconstruction after radiation treatment. Ann Surg 1995;221:756-64.
- Watterson PA, Bostwick J 3rd, Hester TR Jr, et al. TRAM flap anatomy correlated with a 10-year clinical experience with 556 patients. Plast Reconstr Surg 1995;95:1185-94.
- Chang EI, Ly DP, Wey PD. Comparison of aesthetic breast reconstruction after skin-sparing or conventional mastectomy in patients receiving preoperative radiation therapy. Ann Plast Surg 2007;59:78-81.
- Disa JJ, Cordeiro PG, Heerdt AH, et al. Skinsparing mastectomy and immediate autologous tissue reconstruction after whole-breast irradiation. Plast Reconstr Surg 2003;111:118-24.
- Moran SL, Serletti JM, Fox I. Immediate free TRAM reconstruction in lumpectomy and radiation failure patients. Plast Reconstr Surg 2000;106:1527-31.
- Temple CL, Strom EA, Youssef A, et al. Choice of recipient vessels in delayed TRAM flap breast reconstruction after radiotherapy. Plast Reconstr Surg 2005;115:105-13.
- Spear SL, Ducic I, Low M, et al. The effect of radiation on pedicled TRAM flap breast reconstruction: outcomes and implications. Plast Reconstr Surg 2005;115:84-95.
- Chawla AK, Kachnic LA, Taghian AG, et al. Radiotherapy and breast reconstruction: complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys 2002;54:520-6.
- Kuske RR, Schuster R, Klein E, et al. Radiotherapy and breast reconstruction: clinical results and dosimetry. Int J Radiat Oncol Biol Phys 1991;21:339-46.
- Schuster RH, Kuske RR, Young VL, et al. Breast reconstruction in women treated with radiation therapy for breast cancer: cosmesis, complications, and tumor control. Plast Reconstr Surg 1992;90:445-52.
- Calitchi E, Kirova YM, Otmezguine Y, et al. Long-term results of neoadjuvant radiation therapy for breast cancer. Int J Cancer 2001;96:253-9.
- Aryus B, Audretsch W, Gogolin F, et al. Remission rates following preoperative chemotherapy and radiation therapy in patients with breast cancer. Strahlenther Onkol 2000;176:411-5.
- Tran NV, Evans GR, Kroll SS, et al. Postoperative adjuvant irradiation: effects on tranverse rectus abdominis muscle flap breast reconstruction. Plast Reconstr Surg 2000;106:313-7.
- Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg 2002;109:1919-24.
- Williams JK, Carlson GW, Bostwick Jr, et al. The effects of radiation treatment after TRAM flap breast reconstruction. Plast Reconstr Surg 1997;100:1153-60.
- Zimmerman RP, Mark RJ, Kim AI, et al. Radiation tolerance of transverse rectus abdominis myocutaneousfree flaps used in immediate breast reconstruction. Am J Clin Oncol 1998;21:381-5.
- Huang CJ, Hou MF, Lin SD, et al. Comparison of local recurrence and distant metastases between breast cancer patients after postmastectomy radiotherapy with and without immediate TRAM flap reconstruction. Plast Reconstr Surg 2006;118:1079-86.
- Halyard MY, McCombs KE, Wong WW, et al. Acute and chronic results of adjuvant radiotherapy after mastectomy and Transverse Rectus Abdominis Myocutaneous (TRAM) flap reconstruction for breast cancer. Am J Clin Oncol 2004;27:389-94.
- Mehta VK, Goffinet D. Postmastectomy radiation therapy after TRAM flap breast reconstruction. Breast J 2004;10:118-22.
- Hanks SH, Lyons JA, Crowe J, et al. The acute effects of postoperative radiation therapy on the transverse rectus abdominis myocutaneous flap used in immediate breast reconstruction. Int J Radiat Oncol Biol Phys 2000;47:1185-90.
- Hunt KK, Baldwin BJ, Strom EA, et al. Feasibility of postmastectomy radiation therapy after TRAM flap breast reconstruction. Ann Surg Oncol 1997;4:377-84.
- Toth BA, Forley BG, Calabria R. Retrospective study of the skin-sparing mastectomy in breast reconstruction. Plast Reconstr Surg 1999;104:77-84.
- Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg 1991;87:1048-53.
- McCraw JB, Horton CE, Grossman JA, et al. An early appraisal of the methods of tissue expansion and transverse rectus abdominis musculocutaneous flap in reconstruction of the breast following mastectomy. Ann Plast Surg 1987;18:93-113.
- Kroll SS, Baldwin B. A comparison of outcomes using three different methods of breast reconstruction. Plast Reconstr Surg 1992;90:455-62.
- Tadiparthi S, Alrawi M, Collis N. Two-stage delayed breast reconstruction with an expander and free abdominal tissue transfer: Outcomes of 65 consecutive cases by a single surgeon. J Plast Reconstr Aesthet Surg 2011;64:1608-12.
- Georgiade GS, Riefkohl R, Cox E, et al. Long-term clinical outcome of immediate reconstruction after mastectomy. Plast Reconstr Surg 1985;76:415-20.
- Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg 2000;105:930-42.
- Rosen PB, Jabs AD, Kister SJ. Clinical experience with immediate breast reconstruction using tissue expansion or transverse rectus abdominis musculocutaneous flaps. Ann Plast Surg 1990;25:249-57.
- Carlson GW, Bostwick Jr, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg 1997;225:570-5.
- Carlson GW, Losken A, Moore B, et al. Results of immediate breast reconstruction after skin-sparing mastectomy. Ann Plast Surg 2001;46:222-8.
- Cederna PS, Yates WR, Chang P, et al. Postmastectomy reconstruction: comparative analysis of the psychosocial, functional, and cosmetic effects of transverse rectus abdominis musculocutaneous flap versus breast implant reconstruction. Ann Plast Surg 1995;35:458-68.
- Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayedimmediate breast reconstruction. Plast Reconstr Surg 2004;113:1617-28.
- Deurloo EE, Tanis PJ, Gilhuijs KG, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer 2003;39:1068-73.
- Motomura K, Nagumo S, Komoike Y, et al. Intraoperative imprint cytology for the diagnosis of sentinel node metastases in breast cancer. Breast Cancer 2007;14:350-3.
- Creager AJ, Geisinger KR, Shiver SA, et al. Intraoperative evaluation of sentinel lymph nodes for metastatic breast carcinoma by imprint cytology. Mod Pathol 2002;15:1140-7.
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24.
- Israeli R, Feingold RS. Acellular dermal matrix in breast reconstruction in the setting of radiotherapy. Aesthet Surg J 2011;31:51S-64S.
- Panettiere P, Marchetti L, Accorsi D. The serial free fat transfer in irradiated prosthetic breast reconstructions. Aesthetic Plast Surg 2009;33:695-700.
- de Blacam C, Momoh AO, Colakoglu S, et al. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plast Reconstr Surg 2011;128:411e-8e.
- Losken A, Pinell XA, Sikoro K, et al. Autologous fat grafting in secondary breast reconstruction. Ann Plast Surg 2011;66:518-22.
- Salgarello M, Visconti G, Farallo E. Autologous fat graft in radiated tissue prior to alloplastic reconstruction of the breast: report of two cases. Aesthetic Plast Surg 2010;34:5-10.
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007;119:1409-22; discussion 1423-4.
- Panettiere P, Accorsi D, Marchetti L, et al. Largebreast reconstruction using fat graft only after prosthetic reconstruction failure. Aesthetic Plast Surg 2011;35:703-8.
- Phulpin B, Gangloff P, Tran N, et al. Rehabilitation of irradiated head and neck tissues by autologous fat transplantation. Plast Reconstr Surg 2009;123:1187-97.
- Salgarello M, Visconti G, Barone-Adesi L. Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plast Reconstr Surg 2012;129:317-29.


