Current opinions on indications and algorithms for acellular dermal matrix use in primary prosthetic breast reconstruction
Introduction
Since Salzberg’s first description (1) and Breuing’s subsequent publication (2) of the use of human acellular dermis as an adjunct to traditional prosthetic breast reconstruction, there has been a surge of interest in this technique. Today, acellular dermal matrix (ADM) is used in nearly 60 percent of the 50,000 prosthetic-based breast reconstructions that are performed annually (3). Users have cited numerous benefits of ADM in prosthetic breast reconstruction, ranging from better control of anatomic placement (1,4-10) increased definition of the inframammary fold (1,4-10) reduced capsular contracture (5,7-17) faster and fewer expansions especially of the lower pole (1,4,7-10,12,14,16-22) and overall improved aesthetic outcomes (6,23-26).
Nonetheless, important concerns have arisen surrounding ADM use, most notably including increased cost and heightened complication rates (3,8,9,27-31). Among the literature there is a very high variation in many of the reported outcomes for ADM compared to traditional non-ADM techniques (32,33). A literature review by Kim et al. revealed reported complication rates from ADM-assisted reconstruction ranging from as low as 8.6% to as high as 19.5% (28). In that study, the complication rate with ADM use had a pooled average of 15.4%, slightly but statistically significantly higher than the non-ADM pooled average of 14.0%. Certainly, rigorous randomized controlled trials (RCTs) investigating complication rates would be enlightening, but unfortunately only one has been completed to our knowledge; it showed equal outcomes between ADM use and traditional, no-ADM breast reconstruction (30), although the conclusions of this study may be limited by its use of an older, now-disfavored size of ADM sheet (34). The Multi Center Canadian Acellular Dermal Matrix Trial (MCCAT) promises to provide further RCT data to answer this question (35), which is reassuring because clearly, more data from RCTs can help to sharpen our understanding of ADM’s effects on surgical outcomes.
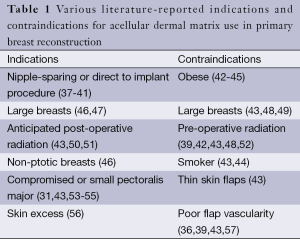
Even so, some have posited that at least some of the disparity in reported complication rates with and without ADM is actually due to the lack of clear indications and contraindications to use (36). The disparate complication rates we are seeing in the literature may be caused by implicit variations in patient selection. Careful and rational patient selection is critical to maximize reconstructive outcomes (9). The literature is rife with associations between certain patient parameters and better or worse outcomes (Table 1). For example, a recent publication by Mendenhall et al. (42) studying the difference between two different ADMs notably found that radiation therapy, larger tissue expander size, and obesity were all predictors of a longer time spent with drains, which itself was associated with a greater number of complications.
Full table
When surgical decision-making relies on many factors, it is common and helpful to develop rational algorithms to help guide decisions. For example, the decision to choose a particular breast reconstruction modality, i.e., prosthetic versus autologous reconstruction, has enjoyed significant discourse that explores rational algorithms (37). Yet until very recently, there has been a paucity of literature on whether selective use of ADM is even possible, let alone specific proposals for algorithms governing ADM use. We believe that the body of literature surrounding ADM indications and contraindications in primary prosthetic breast reconstruction has matured sufficiently to where a discussion of selective ADM algorithms is both now possible and necessary. Therefore, we wish to review the literature’s current opinion on those various indications and contraindications, and discuss the few algorithms that have thus far been proposed.
Pre-operative indications and contraindications
Surgical technique: direct-to-implant and nipple-sparing surgical techniques
The advent of ADM has made major impacts on breast reconstruction, one of the most notable of these being the expanded use of the direct-to-implant reconstructive modality (37,38). These single-stage techniques avoid the need for the expansion visits necessary for tissue expander reconstructions. Because there is no planned expansion of the skin envelope, maximal placement of volume must be achieved at the time of prosthesis insertion. However, the patient’s pectoralis major muscle will likely be insufficient to accommodate the prosthesis, so ADM emerges as an obvious choice to allow the release and augmentation of the existing pectoralis (39).
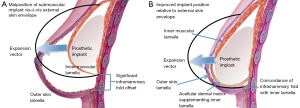
A similar situation arises in the setting of a nipple-sparing mastectomy (NSM) and total-skin-sparing mastectomy (TSSM). In these cases, there will be maximal skin remaining since the surgeon has taken none; hence the discordance between the inner pectoralis lamellae and the outer lamellae of the skin envelope will be high. Proper alignment between these two lamellae improves the overall outcome and aesthetics of the reconstruction (36) (Figure 1). Like in the case of direct-to-implant reconstructions, NSM and TSSM techniques may benefit from releasing the pectoralis muscle and using ADM as a sling augmenting the inner tissue plane (40,41).
Body mass index and breast size
The relationship between body mass index and breast size can affect the concordance between the reconstructive outer lamella and the reconstructive inner lamella. When patients with high body mass index also have large, ptotic breasts, the available pectoralis major muscle may not accommodate the redundant skin hosted by the outer lamella. Therefore, in these patients ADM can be useful in providing the needed inner lamellar surface area, resulting in better contact between the skin flap and the underlying pectoralis muscle layer and ultimately reduced dead space (Figure 1). The ability to manipulate the ADM also confers control over the projection vector of the lower so that it better replicates the shape of the pre-mastectomy breast (46,47). ADM may also be used advantageously in high body mass patients to reduce the risk of seroma and other complications when it is employed as a tool to define the lateral and inferior edges of the prosthetic pocket (36).
On the other hand, it has also been noted that patients with high body mass index and large breasts may be at increased risk of poor wound healing due to the long flaps of skin which become prone to ischemia (43). Some studies have identified obesity as a risk factor for breast reconstruction using ADM (48,49). This conflict in the literature is reflected in Table 1. Therefore, while the use of ADM may help protect the reconstruction in the case of an ischemic complication, a surgeon will likely have to weigh the option of a delayed reconstruction in high body mass index patients with large breasts.
Pre-operative radiation therapy and expectation of post-operative radiation
Pre-operative radiation exposure has been tenuously linked to poor outcomes, most likely due to the observation that irradiation compromises tissue microvasculature by provoking fibrosis (43,58-60). Additionally, fibroblast infiltration into the ADM disrupts its usual incorporation into surrounding tissue. Consequently, the integration of the ADM is threatened by serious complications such as infection, seroma, infection, explantation, and poor aesthetic outcomes because it begins to act as a foreign body (60-62).
But while pre-operative radiation contraindicates the use of ADM, preliminary evidence in conjunction with our own clinical experience suggest that ADM can accelerate expansion of the prosthetic pocket before the start of post-operative radiotherapy and provide protection against the risk of skin flap necrosis induced by post-operative radiation (50). Moreover, when the inferior border of the pectoralis major is released during the course of breast reconstruction with ADM, pectoralis tightness due to post-operative radiation-induced fibrosis is alleviated. For example, post-operatively irradiation was shown to reduce rates of explantation in a study of 428 breasts undergoing two-stage prosthetic breast reconstruction using ADM, compared to historically reported values (62). Likewise, another study reported that postmastectomy radiation therapy without the use of ADM during reconstruction resulted in a 2.63-fold increase in complications, compared to a similar cohort who received ADM during reconstruction (63). In contrast, ADM has not demonstrated any benefits when used on breasts that have undergone pre-operative radiotherapy (39,43,64,65). Therefore, when post-operative radiotherapy is expected, the surgeon should consider using ADM during the breast reconstruction.
Intra-operative indications and contraindications
Pectoralis major anatomy
Surgeons conducting breast reconstruction must continuously assess the integrity and tautness of the pectoralis major as it is being manipulated. During the operation, the pectoralis, serratus fascia, or rectus fascia may be subject to iatrogenic damage that compromises their ability to house the reconstructive pocket. In these cases, ADM is used as an interpositional graft (31,43,47,53,54). On a similar note, expansion of the inferior pole is restricted when the pectoralis muscle is too tight, a situation that can arise when the muscle is too narrow or sits high on the chest wall; therefore, in these cases the pectoralis is released inferiorly and its coverage augmented with ADM in order to close the gap between the inframammary fold and the inferior border of the muscle. Indeed, Madsen et al. reported that 72 percent of patients have pectoralis that is either too high or too narrow (55).
While it is true that these cases may be handled simply with elevation of the serratus and rectus fascial flaps, use of ADM often provides more powerful control over the breast mound. It permits full expansion of the lower pole and limits iatrogenic damage to the serratus anterior and rectus abdominus fascia that would otherwise endanger the definition of the inframammary fold.
Flap vascularity and skin excess
Flap vascularity is a critical prerequisite for expedient wound healing. In the face of numerous, often-conflicting indications and contraindications (Table 1), flap vascularity is in general the most important factor and ought to take precedence in the event of a conflict between different decision-points (36). Skin flaps are inherently hypovascular, which renders them vulnerable to incisional breakdown and prosthetic exposure if they are not managed properly (57). A number of patient comorbidities, notably smoking, peripheral vascular disease, and hypertension have been shown to compromise flap vascularity (57,66,67). The surgeon must observe flap vascularity intra-operatively to assess the degree of perfusion that the flap will be capable of providing. Compromised skin edges must be trimmed for optimal healing. If the flap is significantly devascularized, the subdermal vascular plexus damaged, or the flap itself is too thin, use of ADM is contraindicated due to the probability that it will not integrate (39,43).
If vascularity is deemed sufficient, the decision to use ADM rests on an intra-operative determination of relative skin excess. The goal of the operation is to match the length of the outer skin to the length of the muscle layer underneath, maximizing contact between the two layers. If the pectoralis muscle and the outer skin flap are mismatched, the skin flap may experience tension that can harm the natural aesthetic of the breast as well as disrupt perfusion to the nipple, causing nipple necrosis (56).
Sentinel-node status
As mentioned previously, the expectation of post-operative radiation is a good indicator for ADM use in breast reconstruction because of its beneficial effects on capsular constriction elicited by radiation. From an intra-operative finding of positive lymph node status, the surgeon can infer that the patient will likely undergo post-operative radiation therapy. Therefore such a finding is also a good indicator for the surgeon to use ADM (36).
Previously proposed algorithms
Presently, algorithms that assist surgeons to decide whether ADM is appropriate have not been well investigated, and specific proposals of such algorithms in the literature are sparse. Most such algorithms proposed so far have been one- or two-factor decision tools. For example, Colwell et al. suggested using a focused algorithm for ADM use that centered on the evaluation of skin flap vascularity (39). According to this algorithm, patients with thin or devascularized skin flaps would be reconstructed using total muscular coverage while thick and well-vascularized skin flaps would be reconstructed with ADM using a direct-to-implant technique.
Dupin et al. has also formulated a strategy that helps to identify scenarios where ADM is appropriate (68). In their algorithm, reconstruction assisted by ADM is favored for patients who have small breasts. Patients with large breasts, or those who smoke or are obese, may still be reconstructed with ADM at the surgeon’s discretion, but the algorithm recommends against it if the mastectomy is associated with radiation.
Peled et al. (50) went further by formally assessing their simple algorithm, which selected the use of ADM for patients undergoing total skin-sparing mastectomies whose skin flaps weren’t substantial enough to adequately fill tissue-expanders. Compared to not using ADM at all, which had an infection rate of 27.8%, and compared to indiscriminant ADM use, which had an infection rate of 20%, implementation of their selection algorithm significantly reduced infection rates to 15.8%.
Finally, we have previously published our own algorithm (36) designed to account for a multitude of patient factors and help generate a decision about whether reconstruction should be assisted by ADM. Our algorithm considered patient body mass index, pectoralis anatomy, flap vascularity, skin excess, and pre- and post-operative radiation therapy including sentinel-node status as a predictor of radiation. By deploying the algorithm for consistent use in our practice, we demonstrated a reduction in ADM usage without compromising safety or aesthetic outcomes.
The promises of well-designed algorithms to help ADM decision-making
Cost reductions
Rapidly mounting costs of health care alongside changes brought by the Affordable Care Act are forcing physicians to reevaluate cost-benefit analysis of many procedures, as even the smallest inefficiencies will likely come under close scrutiny. Surgical decision-making should not only include consideration of utility and complications, but must also be sensitive to the economic consequences of the decisions. The economics of ADM use have been hotly debated in the literature, yielding various viewpoints and conclusions. Besides improving outcomes, one benefit of introducing algorithms for ADM use to surgical practice may be a reduction in healthcare costs as a direct result of more selective and therefore less frequent ADM use. Because ADM is instrumental in direct to implant reconstructions, which obviate the second operation of a two-stage reconstruction, ADM makes available a significantly cost-saving option (39,69-72). Even when patients elect to perform further aesthetic procedures after a direct to implant ADM-assisted breast reconstruction, the total cost may still lie below that of traditional non-ADM two-stage techniques (71). de Blacam et al. (69) published a cost-minimization analysis in the United States healthcare setting that estimated the cost of direct to implant reconstruction with ADM to be $5,423.02 versus $10,934 for the traditional non-ADM two-stage technique. This finding makes suggests that algorithms should include surgical technique, such as direct-to-implant, as a relevant factor in deciding ADM appropriateness.
Of course, not every patient is a candidate for a single-stage breast reconstruction, and the more challenging question is whether ADM use in two-stage techniques is still economically sound compared to similar non-ADM two-stage techniques. As an example of the divergent views in the existing literature, de Blacam et al.’s analysis (69) reported that ADM use in two-stage reconstructions cost $11,255, compared to $10,934 for a similar non-ADM option; Krishnan et al. arrived at a similar result, estimating a $362 cost increase when ADM is used. Both of these reported price differences could be described as negligible. In contrast, Bank et al. (73) found a $3,047 increase when ADM was used in two-stage reconstruction, nearly ten times greater than de Blacam’s or Krishnan’s estimation. Some authors have also argued that alternatives to ADM such as dermal allografts provide the same benefits at a reduced price (74-76).
There are many variables that may help or harm the cost-effectiveness of ADM in two-stage reconstruction. ADM use in tissue-expander surgery can cut the number of post-operative expansion visits required because larger intraoperative fill volumes can be attained; in that respect, they have a cost-saving advantage versus a similar non-ADM-assisted technique (71-73). Specifically Bank et al. (73) argued that the cost of breast reconstruction rises in part due to the number of expansion visits needed, and that ADM use in large breasts (over 500 mL) was particularly effective at reducing costs. This analysis seems to support the inclusion of breast size in an ADM-use algorithm, as previously discussed, large breasts also come with countervailing effects that may warrant a delayed reconstruction in such breasts instead (Table 1). But likely the source of uncertainty in the costs of ADM use is driven by its aforementioned variance in reported complication rates (69,77). If ADM’s complication rates are and severities are truly high, the overall cost of using them will obviously increase. Thus, an algorithm whose rational selection of ADM use can successfully reduce complication rates will likely improve the cost-effectiveness of ADM.
Despite these differences and uncertainties, Bank et al. and Krishnan et al. both have suggested that in reality, material costs are the largest factor driving ADM cost-utility analyses (73,75). Presently the market price of a 6 cm × 16 cm sheet of ADM is approximately $3,100. With these assumptions, we reported that our algorithm could save on material costs by $150,000 over a year of 100 prosthetic breast reconstructions, given that our algorithm reduced ADM use from 84% to 36% (36). If material costs truly represent the majority of the economic burden of ADM-use in breast reconstruction, an algorithm that can be more selective in choosing patients for whom to use ADM would represent a substantial improvement in cost-effective surgical practice.
Aesthetic outcomes and complication rates
ADM used in breast reconstruction effectively acts as a supporting hammock for the prosthetic in order to recreate natural breast morphology (47), and many authors have supported claims that it produces better aesthetic outcomes. Vardanian et al. (11) showed ADM can improve the surgeon’s control over and definition of the inframammary fold, boosting aesthetic outcomes. Nguyen et al. (78) comparably demonstrated improved breast mound volume, breast mound placement, and inframammary fold definition with the use of ADM compared to non-ADM-assisted reconstructions. More recently, Forsberg et al. (26) found that ADM resulted in better aesthetic scores in the naturalness of the contour, the symmetry of shape and size, the position on the chest wall, and the overall aesthetic of the reconstructed breast. With all of the positive reports, it is natural to suppose that a mature, honed algorithm can improve reconstruction aesthetic outcomes if it is able to accurately select patients that will benefit most from ADM.
Likewise, such an algorithm can reduce complication rates by allowing surgeons to judiciously decide whether ADM is appropriate given a particular patient’s individual factors. Peled et al. was able to lower complication rates using their basic ADM algorithm for TSSM reconstructions (50), which is an encouraging finding for those interested in further developing more complex algorithms.
The challenge, of course, is developing and refining the right algorithm. For any of these promises to be realized, much greater effort must be applied toward the development and assessment of sophisticated algorithms—ones that account for the many factors the field has identified as indications and contraindications. Furthermore, long-term outcomes such as capsular contracture, as well as detailed aesthetic outcomes, have been insufficiently assessed in ADM algorithms including our own. In order to discover what parameters the surgeon can leverage to make good ADM decisions, researchers must be prepared to conduct long-term studies to assess complications, as well as test for aesthetic end-points in sophisticated manners.
Finally, we wish to stress that it is not our intention to lock surgical decision-making into a rigid decision-making protocol. Obviously, the clinical experiences of different surgical practices may conflict with our algorithm. Furthermore, many situations cannot be perfectly captured by an uncompromising decision algorithm; additional judgment extending past our simple algorithm must also contribute to the decisions in such cases. Rather, the purpose of this algorithm is to stimulate a shift away from the previously indiscriminate use of ADM in breast reconstruction, and encourage discussion about rational selection criteria for patients who would most benefit from ADM.
Conclusions
Surgical decision-making with ADM in primary prosthetic breast reconstruction continues to evolve. We have reviewed some important indications and contraindications for the use of ADM, as well as the few algorithms that have been thus far proposed to assist in the decision of whether ADM is appropriate. This approach can reduce costs and improve aesthetic outcomes and complication rates. We encourage plastics practices to further develop and evaluate their own decision-making tools for ADM use.
Acknowledgements
Authors’ contributions: Statement of authorship: all of the authors contributed significantly to the development of this manuscript. John Y. S. Kim contributed manuscript preparation, literature review.
Funding: Dr. Kim receives funding from the Musculoskeletal Transplant Foundation.
Disclosure: Dr. Kim is a consultant for the Musculoskeletal Transplant Foundation. Michael Vu declares no conflict of interest.
References
- Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1-5. [PubMed]
- Breuing KH, Warren SM. Immediate bilateral breast reconstruction with implants and inferolateral AlloDerm slings. Ann Plast Surg 2005;55:232-9. [PubMed]
- Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346-56. [PubMed]
- Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 2008;32:418-25. [PubMed]
- Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg 2007;120:373-81. [PubMed]
- Topol BM, Dalton EF, Ponn T, et al. Immediate single-stage breast reconstruction using implants and human acellular dermal tissue matrix with adjustment of the lower pole of the breast to reduce unwanted lift. Ann Plast Surg 2008;61:494-9. [PubMed]
- Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg 2007;60:1214-8. [PubMed]
- Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 2009;124:1735-40. [PubMed]
- Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg 2010;125:429-36. [PubMed]
- Becker S, Saint-Cyr M, Wong C, et al. AlloDerm versus DermaMatrix in immediate expander-based breast reconstruction: a preliminary comparison of complication profiles and material compliance. Plast Reconstr Surg 2009;123:1-6; discussion 107-8. [PubMed]
- Vardanian AJ, Clayton JL, Roostaeian J, et al. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg 2011;128:403e-410e. [PubMed]
- Bellows CF, Albo D, Berger DH, et al. Abdominal wall repair using human acellular dermis. Am J Surg 2007;194:192-8. [PubMed]
- Buck DW 2nd, Heyer K, Wayne JD, et al. Diagnostic dilemma: acellular dermis mimicking a breast mass after immediate tissue expander breast reconstruction. Plast Reconstr Surg 2009;124:174e-6e. [PubMed]
- Buinewicz B, Rosen B. Acellular cadaveric dermis (AlloDerm): a new alternative for abdominal hernia repair. Ann Plast Surg 2004;52:188-94. [PubMed]
- Eppley BL. Experimental assessment of the revascularization of acellular human dermis for soft-tissue augmentation. Plast Reconstr Surg 2001;107:757-62. [PubMed]
- Kim JY, Buck DW 2nd, Kloeters O, et al. Reconstruction of a recurrent first dorsal web space defect using acellular dermis. Hand (N Y) 2007;2:240-4. [PubMed]
- Kim JY, Bullocks JM, Basu CB, et al. Dermal composite flaps reconstructed from acellular dermis: a novel method of neourethral reconstruction. Plast Reconstr Surg 2005;115:96e-100e. [PubMed]
- Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg 2005;116:1263-75; discussion 1276-7. [PubMed]
- Chaplin JM, Costantino PD, Wolpoe ME, et al. Use of an acellular dermal allograft for dural replacement: an experimental study. Neurosurgery 1999;45:320-7. [PubMed]
- Heyer K, Buck DW 2nd, Kato C, et al. Reversed acellular dermis: failure of graft incorporation in primary tissue expander breast reconstruction resulting in recurrent breast cellulitis. Plast Reconstr Surg 2010;125:66e-68e. [PubMed]
- Losken A. Early Results Using Sterilized Acellular Human Dermis (Neoform) in Post-Mastectomy Tissue Expander Breast Reconstruction. Plast Reconstr Surg 2009. [Epub ahead of print]. [PubMed]
- Spear SL, Carter ME, Ganz JC. The correction of capsular contracture by conversion to "dual-plane" positioning: technique and outcomes. Plast Reconstr Surg 2003;112:456-66. [PubMed]
- Gurunluoglu R, Gurunluoglu A, Williams SA, et al. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg 2013;70:103-10. [PubMed]
- Namnoum JD. Expander/implant reconstruction with AlloDerm: recent experience. Plast Reconstr Surg 2009;124:387-94. [PubMed]
- Rawlani V, Buck DW 2nd, Johnson SA, et al. Tissue expander breast reconstruction using prehydrated human acellular dermis. Ann Plast Surg 2011;66:593-7. [PubMed]
- Forsberg CG, Kelly DA, Wood BC, et al. Aesthetic outcomes of acellular dermal matrix in tissue expander/implant-based breast reconstruction. Ann Plast Surg 2014;72:S116-20. [PubMed]
- Antony AK, McCarthy CM, Cordeiro PG, et al. Acellular human dermis implantation in 153 immediate two-stage tissue expander breast reconstructions: determining the incidence and significant predictors of complications. Plast Reconstr Surg 2010;125:1606-14. [PubMed]
- Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:28-41. [PubMed]
- Lanier ST, Wang ED, Chen JJ, et al. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg 2010;64:674-8. [PubMed]
- McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 2012;130:57S-66S. [PubMed]
- Weichman KE, Wilson SC, Weinstein AL, et al. The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction. Plast Reconstr Surg 2012;129:1049-58. [PubMed]
- Winters ZE, Colwell AS. Role of acellular dermal matrix-assisted implants in breast reconstruction. Br J Surg 2014;101:444-5. [PubMed]
- Rundell VL, Beck RT, Wang CE, et al. Complication prevalence following use of tutoplast-derived human acellular dermal matrix in prosthetic breast reconstruction: a retrospective review of 203 patients. J Plast Reconstr Aesthet Surg 2014;67:1345-51. [PubMed]
- Nahabedian MY. Discussion: The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg 2012;130:67S-9S. [PubMed]
- Zhong T, Temple-Oberle C, Hofer S, et al. The Multi Centre Canadian Acellular Dermal Matrix Trial (MCCAT): study protocol for a randomized controlled trial in implant-based breast reconstruction. Trials 2013;14:356. [PubMed]
- Jordan SW, Khavanin N, Fine NA, et al. An algorithmic approach for selective acellular dermal matrix use in immediate two-stage breast reconstruction: indications and outcomes. Plast Reconstr Surg 2014;134:178-88. [PubMed]
- Ballard TN, Momoh AO. Advances in breast reconstruction of mastectomy and lumpectomy defects. Surg Oncol Clin N Am 2014;23:525-48. [PubMed]
- Colwell AS. Current strategies with 1-stage prosthetic breast reconstruction. Gland Surg 2015;4:111-5. [PubMed]
- Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg 2011;128:1170-8. [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [PubMed]
- Sbitany H, Wang F, Peled AW, et al. Tissue Expander Reconstruction After Total Skin-Sparing Mastectomy: Defining the Effects of Coverage Technique on Nipple/Areola Preservation. Ann Plast Surg 2014. [Epub ahead of print]. [PubMed]
- Mendenhall SD, Anderson LA, Ying J, et al. The BREASTrial: stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction. Plast Reconstr Surg 2015;135:29e-42e. [PubMed]
- Nahabedian MY. Acellular dermal matrices in primary breast reconstruction: principles, concepts, and indications. Plast Reconstr Surg 2012;130:44S-53S. [PubMed]
- Gubitosi A, Docimo G, Parmeggiani D, et al. Acellular bovine pericardium dermal matrix in immediate breast reconstruction after Skin Sparing Mastectomy. Int J Surg 2014;12 Suppl 1:S205-8. [PubMed]
- Ibrahim AM, Shuster M, Koolen PG, et al. Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg 2013;132:1057-66. [PubMed]
- Ganske I, Verma K, Rosen H, et al. Minimizing complications with the use of acellular dermal matrix for immediate implant-based breast reconstruction. Ann Plast Surg 2013;71:464-70. [PubMed]
- Spear SL, Sher SR, Al-Attar A. Focus on technique: supporting the soft-tissue envelope in breast reconstruction. Plast Reconstr Surg 2012;130:89S-94S. [PubMed]
- Lardi AM, Ho-Asjoe M, Mohanna PN, et al. Immediate breast reconstruction with acellular dermal matrix: factors affecting outcome. J Plast Reconstr Aesthet Surg 2014;67:1098-105. [PubMed]
- Gdalevitch P, Ho A, Genoway K, et al. Direct-to-implant single-stage immediate breast reconstruction with acellular dermal matrix: predictors of failure. Plast Reconstr Surg 2014;133:738e-747e. [PubMed]
- Peled AW, Foster RD, Garwood ER, et al. The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study. Plast Reconstr Surg 2012;129:901e-908e. [PubMed]
- Moyer HR, Pinell-White X, Losken A. The effect of radiation on acellular dermal matrix and capsule formation in breast reconstruction: clinical outcomes and histologic analysis. Plast Reconstr Surg 2014;133:214-21. [PubMed]
- Hille-Betz U, Kniebusch N, Wojcinski S, et al. Breast reconstruction and revision surgery for implant-associated breast deformities using porcine acellular dermal matrix: a multicenter study of 156 cases. Ann Surg Oncol 2015;22:1146-52. [PubMed]
- Henseler H, Smith J, Bowman A, et al. Subjective versus objective assessment of breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:634-9. [PubMed]
- Kim JY, Connor CM. Focus on technique: two-stage implant-based breast reconstruction. Plast Reconstr Surg 2012;130:104S-15S. [PubMed]
- Madsen RJ Jr, Chim J, Ang B, et al. Variance in the origin of the pectoralis major muscle: implications for implant-based breast reconstruction. Ann Plast Surg 2015;74:111-3. [PubMed]
- Gould DJ, Hunt KK, Liu J, et al. Impact of surgical techniques, biomaterials, and patient variables on rate of nipple necrosis after nipple-sparing mastectomy. Plast Reconstr Surg 2013;132:330e-8e. [PubMed]
- Seth AK, Hirsch EM, Fine NA. Additive risk of tumescent technique in patients undergoing mastectomy with immediate reconstruction. Ann Surg Oncol 2011;18:3041-6. [PubMed]
- Clemens MW, Kronowitz SJ. Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review. Plast Reconstr Surg 2012;130:27S-34S. [PubMed]
- Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg 2004;113:877-81. [PubMed]
- Fine NA, Hirsch EM. Keeping options open for patients with anticipated postmastectomy chest wall irradiation: immediate tissue expansion followed by reconstruction of choice. Plast Reconstr Surg 2009;123:25-9. [PubMed]
- Parks JW, Hammond SE, Walsh WA, et al. Human acellular dermis versus no acellular dermis in tissue expansion breast reconstruction. Plast Reconstr Surg 2012;130:739-46. [PubMed]
- Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plast Reconstr Surg 2012;130:1-9. [PubMed]
- Seth AK, Hirsch EM, Fine NA, et al. Utility of acellular dermis-assisted breast reconstruction in the setting of radiation: a comparative analysis. Plast Reconstr Surg 2012;130:750-8. [PubMed]
- Dubin MG, Feldman M, Ibrahim HZ, et al. Allograft dermal implant (AlloDerm) in a previously irradiated field. Laryngoscope 2000;110:934-7. [PubMed]
- Weichman KE, Cemal Y, Albornoz CR, et al. Unilateral preoperative chest wall irradiation in bilateral tissue expander breast reconstruction with acellular dermal matrix: a prospective outcomes analysis. Plast Reconstr Surg 2013;131:921-7. [PubMed]
- Goodwin SJ, McCarthy CM, Pusic AL, et al. Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg 2005;55:16-19; discussion 19-20. [PubMed]
- McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg 2008;121:1886-92. [PubMed]
- Dupin C, Mayo J, Hogan M. A History of Breast Reconstruction Following Mastectomy. In: Riker AI. editor. Breast Disease. New York: Springer Science+Business Media, 2015:253-56.
- de Blacam C, Momoh AO, Colakoglu S, et al. Cost analysis of implant-based breast reconstruction with acellular dermal matrix. Ann Plast Surg 2012;69:516-20. [PubMed]
- Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part II. A cost analysis. Plast Reconstr Surg 2011;127:2245-54. [PubMed]
- Kilchenmann AJ, Lardi AM, Ho-Asjoe M, et al. An evaluation of resource utilisation of single stage porcine acellular dermal matrix assisted breast reconstruction: A comparative study. Breast 2014;23:876-82. [PubMed]
- Johnson RK, Wright CK, Gandhi A, et al. Cost minimisation analysis of using acellular dermal matrix (Strattice™) for breast reconstruction compared with standard techniques. Eur J Surg Oncol 2013;39:242-7. [PubMed]
- Bank J, Phillips NA, Park JE, et al. Economic analysis and review of the literature on implant-based breast reconstruction with and without the use of the acellular dermal matrix. Aesthetic Plast Surg 2013;37:1194-201. [PubMed]
- Lynch MP, Chung MT, Rinker BD. A Comparison of Dermal Autograft and Acellular Dermal Matrix in Tissue Expander Breast Reconstruction: Long-term Aesthetic Outcomes and Capsular Contracture. Ann Plast Surg 2015;74 Suppl 4:S214-7. [PubMed]
- Krishnan NM, Chatterjee A, Van Vliet MM, et al. A comparison of acellular dermal matrix to autologous dermal flaps in single-stage, implant-based immediate breast reconstruction: a cost-effectiveness analysis. Plast Reconstr Surg 2013;131:953-61. [PubMed]
- Kim YW, Kim YJ, Kong JS, et al. Use of the pectoralis major, serratus anterior, and external oblique fascial flap for immediate one-stage breast reconstruction with implant. Aesthetic Plast Surg 2014;38:704-10. [PubMed]
- Krishnan NM, Chatterjee A, Rosenkranz KM, et al. The cost effectiveness of acellular dermal matrix in expander-implant immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2014;67:468-76. [PubMed]
- Nguyen KT, Mioton LM, Smetona JT, et al. Esthetic Outcomes of ADM-Assisted Expander-Implant Breast Reconstruction. Eplasty 2012;12:e58. [PubMed]


