
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal metastasis from breast cancer: a preliminary report of 4 cases
Introduction
The incidence of breast cancer (BC) ranked first and the fifth highest mortality rate (69.5/100,000) among women in China (1). The 5-year survival rate of BC is over 80% in North American and Japan; however, recurrent BC treatment remains a challenge (2-5). Typical metastasis sites of BC in order of frequency are the bones (67.8%), liver (47.8%), lungs (42.6%), brain (15.2%), and peritoneum (peritoneal carcinomatosis, PC) 7.6%. The prevalence of peritoneal metastases was 0.7% (4,6). Chemotherapy and anti-estrogen therapy are common options in treating breast cancer peritoneal carcinomatosis (BC PC); however, the effects of such treatments are poor.
In this paper, we report 4 cases in which BC PC was successfully treated using a combination of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS + HIPEC) and conduct a literature review to provide new ideas for the treatment of BC PC. The following article is presented following the AME Case Series reporting checklist (available at http://dx.doi.org/10.21037/gs-20-893).
Methods
Clinical information
From January 2015 to March 2020, 893 BC patients underwent radical resection at Beijing Shijitan Hospital; 17 of whom had progressive disease with PC. Of these 17 patients, 4 BC PC patients, who underwent CRS + HIPEC, participated in this retrospective study. The remaining 13 patients with BC PC were excluded as they met the exclusion criteria or did not agree to undergo CRS + HIPEC. All patients’ diagnoses of BC PC were confirmed by pathology.
The Ethical Committee of the Beijing Shijitan Hospital approved the study design (BJSJTH2015-28). All of the patients were provided with detailed information about the CRS + HIPEC process and signed informed consent forms. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The combination of CRS + HIPEC is a standard treatment for PC and has a standard clinical path that includes detailed inclusion and exclusion criteria (7). To be eligible to participate in the study, the patients had to meet the following inclusion criteria: (I) have a clinical picture of BC PC and pathological confirmation; (II) have a Karnofsky performance status score of ≥60; (III) have a normal peripheral blood white blood cell count ≥3.5×109/L, and a platelet count ≥80×109/L; (IV) have an acceptable liver function with bilirubin ≤2× the upper limit of normal (ULN), and aspartic aminotransferase and alanine aminotransferase ≤2× ULN; (V) have an acceptable renal function with serum creatinine ≤1.2× ULN; and (VI) have cardiovascular pulmonary and other major organ functions that could withstand a major operation.
Conversely, patients were excluded from the study if they met the following exclusion criteria: (I) have bone, liver, lung, brain, or other distant metastases; (II) have serum bilirubin, aspartic aminotransferase and alanine aminotransferase levels >2× ULN; (III) have a serum creatinine level >1.2× ULN; (IV) show significant mesenteric in a contracture imaging examination; and/or (V) could not withstand a major operation due to their general status or the functions of their major organs.
The CRS + HIPEC procedure
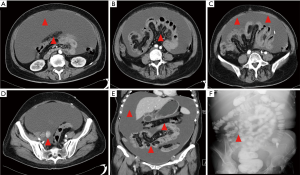
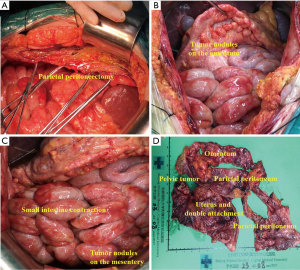
The same professional PC treatment team conducted all CRS + HIPEC. The peritoneal cancer index (PCI) was evaluated based on the nature of the ascites, tumor size, and location after the cutting of the abdominal cavity (8) (see Figure 1A). Subsequently, according to the peritonectomy procedures by Sugarbaker, a curative or palliative resection, peritonectomy, lymphadenectomy, or maximum CRS was conducted (9). The completeness of cytoreduction (CC) was evaluated based on residual tumor size (10) (see Figure 1B). HIPEC was carried out immediately after CRS. The HIPEC regimen consisted of docetaxel 120 mg plus cisplatin 120 mg. HIPEC was conducted using the open Coliseum technique (8). Each drug was added to 3 L saline and heated to 43±0.5 °C. The HIPEC time for each drug was 30 mins, and the flow rate was 400 mL/min. Gastrointestinal tract reconstruction, abdominal drainage tube placement, and tension reduction suture incision were performed after HIPEC (11).
Study endpoint
The primary endpoint was the overall survival (OS) time from CRS + HIPEC. The secondary endpoint was the perioperative safety of CRS + HIPEC in BC PC.
Definition
The following definitions were adopted for this study:
- Overall survival 1 (OS1) was defined as the period from the day of the BC to the day of death or the last follow-up data; Overall survival 2 (OS2) was defined as the period from the day of CRS + HIPEC to the day of death or related with BC PC, or the last follow-up date;
- The perioperative period was defined as 30 days after CRS + HIPEC (11);
- Adverse events (AEs) were defined according to the Clavier-Dindo classification system. Under this system, a Grade I AE is any deviation from the normal postoperative course without the need of a pharmacological treatment or surgical, endoscopic, or radiological intervention; a Grade II AE requires pharmacological treatment with drugs other than that allowed for Grade I complications, or blood transfusions or total parenteral nutrition; a Grade III event requires surgical, endoscopic or radiological interventions; a Grade IV event comprises a life-threatening complication requiring intermediate care or intensive care unit management; a Grade V AE is defined as the death of a patient (12,13).
Follow-up
Patients were followed up every 3 months for the first 2 years and every 6 months after that. Follow-up items included a physical examination, tumor markers, a breast, and gynecological color Doppler ultrasound, and a chest and abdomen computed tomography (CT). At the last follow-up date of March 1, 2020, the rate of follow-up was 100%.
Results
Patients’ major clinicopathological characteristics
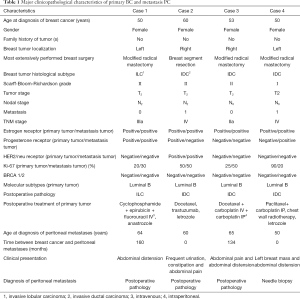
All 4 patients were female. Patients’ average age was 59.8 (range, 50–65) years at CRS + HIPEC. In terms of pathology, 1 patient had invasive lobular carcinoma (ILC), and 3 had invasive ductal carcinoma (IDC). The molecular typing of primary tumors of all 4 cases was Luminal B. Table 1 shows the major clinicopathological characteristics of primary BC and metastasis PC.
Full table
Major characteristics of CRS + HIPEC
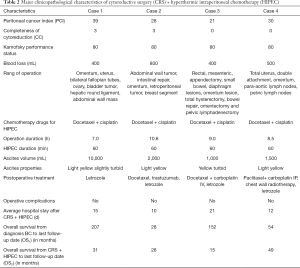
The average time of CRS + HIPEC was 8.8 h (range, 7–10.6 h). The median number of resected organs was 7 (range, 5–9). The average blood loss was 525 mL (range, 400–800 mL). The average ascites volume was 3,625 mL (range, 1,000–10,000 mL). Concerning the HIPEC regimen, all 4 cases were treated with docetaxel 120 mg plus cisplatin 120 mg. The average PCI was 29.5 (range, 21–39). Cases 1 and 2 had CC scores of 0. Cases 3 and 4 had CC scores of 3. Table 2 shows the major clinicopathological characteristics of CRS + HIPEC.
Full table
OS and safety analysis
Overall, following their BC diagnosis, each patient survived 207, 28, 152, and 54 months, respectively (Table 2). Following the CRS + HIPEC treatment, all 4 patients were alive, and their OS periods were 31, 28, 15, and 49 months, respectively.
Case 1 displayed incision liquefaction after the CRS + HIPEC; however, no AEs occurred in the other 3 cases. Further, there were no serious adverse events (SAEs) (AE > Grade III) during the perioperative period.
Discussion
Our study examined the detailed process of CRS + HIPEC and found that patients with BC PC may benefit from this treatment. The results showed that CRS + HIPEC extended BC PC patients’ OS. Further, no SAEs occurred during the CRS + HIPEC perioperative period.
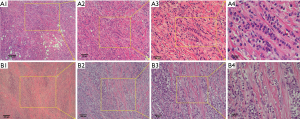
The most common metastasis sites of primary BC with IDC included the regional lymph nodes, lung, liver, bones, brain, and skin. BC with ILC frequently affects the bones, retroperitoneum, peritoneum, gynecological organs, and gastrointestinal tract (14,15). ILC accounts for less than 10% of all BCs, while IDC accounts for more than 90% of all BCs. However, the loss of E-cadherin expression on the surface of tumor cells in patients with ILC leads to more diverse forms of metastasis, and prevents cell adhesion, and promotes tumor cell migration (16). Peritoneal metastasis can be diagnosed by CT or surgery. Only 3% of patients have IDC, while 11% have ILC (P=0.006). Regardless of whether patients have IDC or ILC, PC is a major reason for morbidity and mortality (17). In the present study, 1 patient had ILC, and 3 patients had IDC of the primary BC (Case 1, see Figures 1,2,3). The metastasis tumor and the primary tumor were of the same pathological type.
Patients with a prognosis of PC have poorer survival rates than patients with other regional metastases from BC. The median survival time of patients with BC was 20.5 months from diagnosis of metastasis, while patients with BC PC’s median survival was only 1.5 months (18). A previous study showed that OS was 5.8 months in BC patients with metastasis PC compared to 22.6 months in patients with no metastasis PC from diagnosis. Patients with synchronous metastases had significantly better survival rates than those with metachronous metastases (19). In the present study, 2 patients had metachronous metastases, and 2 had synchronous metastases. The longest OS of patients with synchronous metastases was 49 months, while the longest OS of patients with metachronous metastases was 31 months (as of March 2020) (see Figure 4).

BC PC can cause abdominal distension, abdominal pain, and severe intestinal obstruction. All of the patients in the present study displayed at least one of the above symptoms. However, there were no effective treatments to relieve these symptoms, and chronic malnutrition caused poor prognoses. The traditional treatment methods for BC PC are chemotherapy or radiotherapy, but such treatments’ effects are unsatisfactory. The BC PC treatment in our study was CRS + HIPEC. To achieve radical CRS, the median number of resected organs were 7; 2 patients reached a CC score of 0. The tolerance to hyperthermia was higher in normal tissue than tumor tissue. The synergistic anti-cancer effect can be dramatically increased at 43 °C. Hyperthermia could increase the response rates of cancer cells to HIPEC drugs and HIPEC drugs’ depth into the tumor tissues. Finally, loosening the adhesion of the intestine or ileostomy was shown to relieve the intestinal obstruction. In the present study, 1 patient underwent a procedure to loosen the intestine’s adhesion, and another underwent ileostomy, which completely relieved the abdominal distension or bowel obstruction. All of the patients in the study received adjuvant chemotherapy and endocrine therapy pre- and post-CRS + HIPEC. The median OS of 30 months was better than that cited in the literature.
Estrogen plays an important role in the occurrence and prognosis of BC. The estrogen receptor (ER) is an important biomarker in predicting BC prognosis (20). BC patients with ER- and progesterone receptor (PR)- positive had a better prognosis than patients with ER and PR negative (21). Human epidermal growth factor receptor 2 (HER2) regulates cell proliferation, growth, and survival. HER2 is a transmembrane tyrosine kinase receptor (22,23). BC patients with high levels of Ki-67, which is a nuclear proliferation marker, usually have a poor prognosis (24). In the present study, 2 patients had synchronous BC PC (1 had Ki-67 80–90%, and the other was HER2 positive), leading to early peritoneal metastasis. The other 2 cases received standard adjuvant therapy after the primary lesion. All of the patients received tamoxifen treatment for 5 years, and metastasis occurred after 5 years of discontinuation (all 4 cases had positive ERs). After CRS + HIPEC, it was necessary to administer anti-estrogen therapy to hormone receptor-positive BC PC patients. All 4 patients received letrozole orally after CRS + HIPEC and chemotherapy. No tumor progression was detected at the time of follow up.
The average PCI was 29.5, which indicates the difficulty of CRS. The average operation duration was 8.8 h; 7 organs were resected on average. The average blood loss was 525 mL, and the average ascites volume was 3,625 mL. There were no SAEs during the perioperative period, and the average hospital stay was 15 d. The safety of CRS + HIPEC was accepted. Notably, a professional PC treatment team implemented standardized CRS + HIPEC. Thus, the opposite conclusion that CRS + HIPEC was not the treatment of choice cannot be drawn (25). This article provides new ideas and methods for the treatment of BC PC patients. These findings merit further investigation in studies with larger sample sizes.
All 4 patients were satisfied with the treatment effects, especially that their symptoms associated with abdominal discomfort were relieved, and their quality of life was improved.
One disadvantage of this study is due to the small number of patients; a statistical analysis could not be performed. Additionally, as the follow-up time was short, no comparison could be drawn between the control group, and no questionnaires were administered to evaluate quality of life (QoL). Thus, the findings in this study need to be confirmed in future studies with larger sample sizes.
Conclusions
This paper reported 4 typical BC PC cases that were successfully treated with radical comprehensive treatments of CRS + HIPEC. To date, these patients remain in good condition. Patients’ median OS from CRS + HIPEC was 30 months. The 4 cases provide evidence that an integrated therapy of CRS + HIPEC is a promising strategy that could improve BC PC patients’ outcomes.
Acknowledgments
Funding: This work was supported by the Beijing Municipal Administration of Hospital Ascent Plan (DFL20180701); Beijing Municipal Grant for Medical Talents Group on Peritoneal Surface Oncology (2017400003235J007) (both to Yan Li).
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-893
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-893
Peer Review File: Available at http://dx.doi.org/10.21037/gs-20-893
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-893). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients. All data in the study were analyzed anonymously which approved by Beijing Shijitan Hospital ethical committee (approve number is BJSJTH2015-28). The patients gave written informed consent for the publication of their cases.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Sun YS, Zhao Z, Yang ZN, et al. Risk factors and preventions of breast cancer. Int J Biol Sci 2017;13:1387-97. [Crossref] [PubMed]
- Kouloulias V, Triantopoulou S, Uzunoglou N, et al. Hyperthermia is now included in the NCCN clinical practice guidelines for breast cancer recurrences: an analysis of existing data. Breast Care 2015;10:109-16. [Crossref] [PubMed]
- Bertozzi S, Londero AP, Cedolini C, et al. Prevalence, risk factors, and prognosis of peritoneal metastasis from breast cancer. Springerplus 2015;4:688. [Crossref] [PubMed]
- McLemore EC, Pockaj BA, Reynolds C, et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol 2005;12:886-94. [Crossref] [PubMed]
- Pasqual EM, Bertozzi S, Londero AP, et al. Microscopic peritoneal carcinomatosis in gastric cancer: prevalence, prognosis and predictive factors. Oncol Lett 2018;15:710-6. [PubMed]
- Li Y, Zhou YF, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol 2016;22:6906-16. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol 2001;27:239-43. [Crossref] [PubMed]
- Li XB, Ma R, Ji ZH, et al. Perioperative safety after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal origin: Experience on 254 patients from a single center. Eur J Surg Oncol 2020;46:600-6. [Crossref] [PubMed]
- Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518-26. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Solomayer EF, Diel IJ, Meyberg GC, et al. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat 2000;59:271-8. [Crossref] [PubMed]
- Arrangoiz R, Papavasiliou P, Dushkin H, et al. Case report and literature review: metastatic lobular carcinoma of the breast an unusual presentation. Int J Surg Case Rep 2011;2:301-5. [Crossref] [PubMed]
- Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015;163:506-19. [Crossref] [PubMed]
- Inoue M, Nakagomi H, Nakada H, et al. Specific sites of metastases in invasive lobular carcinoma: a retrospective cohort study of metastatic breast cancer. Breast Cancer 2017;24:667-72. [Crossref] [PubMed]
- Tuthill M, Pell R, Guiliani R, et al. Peritoneal disease in breast cancer: a specific entity with an extremely poor prognosis. Eur J Cancer 2009;45:2146-9. [Crossref] [PubMed]
- Flanagan M, Solon J, Chang KH, et al. Peritoneal metastases from extra-abdominal cancer - a population-based study. Eur J Surg Oncol 2018;44:1811-7. [Crossref] [PubMed]
- Fragomeni S M, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am 2018;27:95-120. [Crossref] [PubMed]
- Oudanonh T, Nabi H, Ennour-Idrissi K, et al. Progesterone receptor status modifies the association between body mass index and prognosis in women diagnosed with estrogen receptor positive breast cancer. Int J Cancer 2020;146:2736-45. [Crossref] [PubMed]
- Kim MH, Kim GM, Kim JH, et al. Intermediate HER2 expression is associated with poor prognosis in estrogen receptor-positive breast cancer patients aged 55 years and older. Breast Cancer Res Treat 2020;179:687-97. [Crossref] [PubMed]
- Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134-50. [Crossref] [PubMed]
- Dumanskiy YV, Bondar OV, Stoliarchuk EA. The Ki-67 marker for assessing the effectiveness of systemic or regional neoadjuvant polychemotherapy in patients with locally advanced breast cancer. Exp Oncol 2019;41:176-8. [Crossref] [PubMed]
- Beniey M. Peritoneal metastases from breast cancer: a scoping review. Cureus 2019;11:e5367 [Crossref] [PubMed]
(English Language Editors: L. Huleatt and J. Chapnick)


