The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes
Introduction
Microvascular anastomotic techniques have evolved considerably since the first vessel anastomosis in 1902. Carrell sutured his anastomosis, and clinicians since then have proceeded to develop ways to build on this paradigm-changing achievement. Through new technology, techniques and treatments, we continue with the aim of improving the quality of care offered through microsurgical reconstructive techniques. Traditionally, vessel anastomosis utilized hand-tied monofilament microsutures, under microscope magnification. There have been developments in suture materials over time, and more recently, the use of microclips or vascular staples have become increasingly used. More popularised still are the use of coupling devices, which use a ring and hooks for microvascular anastomosis. Venous couplers were first described in 1962 by Nakayama, who used an interlocking metal ring with 12 pins to achieve a patent venous anastomosis (1). A reliable device was developed in Sweden in the early 1980’s known as the Unilink coupler and marketed by 3M (2). The technology has been since been refined further and is currently used across the world for microsurgical venous anastomosis as the microvascular anastomotic coupler system coupler system (Synovis Micro Companies Alliance, Birmingham, AL, USA). The venous coupler has been designed to provide intima to intima contact without intraluminal suture material which might act as a site for thrombus promotion.
A systematic review of coupler performance studies demonstrated a thrombosis rate range of 0% to 3%, whilst the average time of using the device is 5 minutes (3). There is sparse published data on cost analysis and the impact of operator experience on the anastomotic coupler device success. Improvements in outcomes other than time benefits have also not been shown. This study aims to address these deficiencies in the literature.
Methods
A retrospective clinical study was undertaken, aiming to compare equivalent groups of patients that had free flap surgery with venous micro-anastomoses with those that had sutured anastomoses. The cohort comprised all patients undergoing microsurgical breast reconstruction at the St Andrew’s Centre for Plastic Surgery & Burns from January 2009 to December 2014. Specific data from all microsurgical free tissue transfer operations were prospectively entered into a clinical database and collected. Key variables collected included patient demographics, operative time, coupler size, surgeon experience and complications. The surgeon level of experience was compared for consultant surgeon versus fellow/registrar; the recipient site was compared for the internal mammary versus the subscapular/thoracodorsal axis; and the number of flaps was compared as to whether a case required a unilateral reconstruction, bilateral reconstructions, a bipedicled flap (two anastomoses in the chest) or stacked flaps (two flaps in series with one anastomosis in the chest).
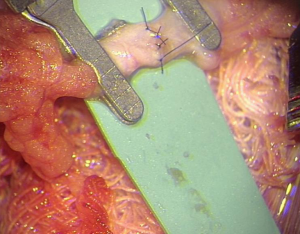
Venous anastomoses were undertaken with either a purely end to end, interrupted suturing technique, or the microvascular anastomotic coupling system (Synovis Micro Companies Alliance, Birmingham, AL, USA). Coupling technique comprised several key steps. Firstly, the use of a vessel sizer to determine the size of the coupler for any individual case, with the vessel end prepared and passed through the coupling ring. The vessel was fixed to the ring by everting the edges over sharp hooks. After each vessel end was prepared in this manner, the coupling device was turned to oppose the rings (Figure 1), and then detached from the ring to leave the completed anastomosis. All arterial anastomoses were performed in an end to end, interrupted sutured fashion (Figure 2).

There were two main outcome measures investigated: anastomotic time and clinical anastomotic failures. Anastomotic time was recorded prospectively, and comprised the time from the end of vessel preparation until removal of vessel clamps. Clinical outcomes assessed were anastomotic failure, returns to theatre and overall flap failure.
Statistical analysis was undertaken using SPSS (SPSS Inc. IBM, Armok, NY, USA). A P value of 0.05 was used to represent statistical significance.
Results
Between January 2010 to December 2014, 1,064 patients underwent 1,206 free flap breast reconstructions. The average age of patients was 50 years. Seventy percent of patients underwent mastectomy and immediate reconstruction during this period with the remaining 30% having a delayed reconstruction. The 1,206 free flaps comprised of 83 transverse myocutaneous upper gracilis (TUG) flaps, and 1,123 deep inferior epigastric artery perforator (DIEP) flaps. In total the coupler was used in 319 flaps, 26% of the cohort.
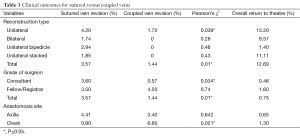
There was a statistically significant clinical benefit in using the anastomotic coupler for venous anastomosis (Table 1). Overall, the return to theatre rate was 12.69% whilst the overall flap loss rate was 0.75%. The overall coupler failure rate was significantly less at 1.4% whilst sutured vein failure rate was 3.57% (P=0.001). Of note, consultant surgeons had a lower coupler failure rate than more junior surgeons (fellows or registrars) at 0.57% versus 4.5%
Full table
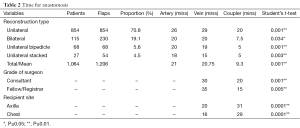
There was also a statistically significant time benefit in using the anastomotic coupler (Table 2). The average time to undertake a sutured vein anastomosis was 21 minutes whilst using the coupler device it was significantly less at 9.3 minutes (P=0.001). Fellows or Registrars took significantly less time to use the coupler at 15 minutes versus sutured vein at 35 minutes (P=0.001). When comparing different recipient sites, it took on average 4 more minutes to use a coupler in the axilla than a coupler in the internal mammary site, and 13 minutes more to suture at the internal mammary site as compared to using a coupler.
Full table
Discussion
Technology in free flap surgery has helped to transition what began as a high risk and significant undertaking in the early 1980’s to routine options in many reconstructive settings. Venous problems are more commonly seen in free flap surgery, and means to counter such problems are eagerly sought. Venous complications requiring a return to theatre and re-anastomosis in sutured anastomosis was 3.57% in our study, and this is commensurate with existing published data on venous complications (4). This contrasts to the venous thrombosis rate achieved with the coupler device of 1.44%, found to be significantly lower than the sutured cohort (P<0.01). Sutured repairs have an inherently higher risk for thrombosis, and are technique dependant on success. Suture material within the lumen, incompletely everted vessel edges and poor suture placement leading to leaks can all contribute to anastomotic failure (5). Blood flow modelling using computational fluid dynamics that assesses flow through a coupler anastomosis versus sutured, show a reduction in the key precursors to thrombin formation, changes in flow velocity profile and decreased wall shear stress (6).
Anastomosis time was significantly quicker with the coupler device. The average anastomosis time of 9 minutes is higher than some other large series in the published literature (3,7), however this was a statistically significant difference to sutured anastomosis, as mirrored in those other publications. Time is an important factor when considering the potential length of a complicated procedure, with increase operative time in microsurgical breast reconstruction associated with lower haemoglobin levels and an increase risk of postoperative complications (8). The cost savings alone are a significant consideration.
Our unit began to routinely use the coupler system in late 2012, although the surgeons involved had all used the system in other hospitals. There was a significant difference in anastomosis times and failure rate in the coupler group when looking at surgeon seniority. Consultant surgeons took longer to use the coupler but had a significantly lower revision rate than fellows or registrars. Fellows and registrars took less time to use a coupler than sutured anastomosis but there was no significant difference in their anastomotic failure rates. This evidence points to a potential learning curve for the use of the coupler device, and that the time saving benefits must be tempered with the knowledge of potential increase in anastomosis revision rate in the hands of a more junior surgeon. This data reinforces the fact that with seniority and experience comes the insight and knowledge that microsurgery should never be rushed.
Within a resource-constrained healthcare system, time is a significant cost factor to consider. The average time difference between sutured and coupler anastomosis was just over 11 minutes. Within our regional hospital system, the financial cost associated with running an operating theatre per hour has been calculated by the hospital as 14 GBP/min. Simplistically, this would indicate that couplers on average save £154. The current cost of the single use couplers excluding the initial investment in coupler set is £169.50. This would indicate that it could be perceived as a cost neutral device. This assumption holds if the time saving allows further use of the fixed costs associated with running a theatre. Given that in our unit the senior author has routinely undertaken three DIEP reconstructions in one theatre in a single day, time saved can be realised as a net cost saving. Furthermore, if you extrapolate the reduction in return to theatre rates which are strongly associated with significant cost increase (9), the net saving for coupler use will increase further. While not a formal cost analysis, this data does point to couplers not being a cost strain.
Coupler use has for the first time been demonstrated in a large cohort to be quicker not only in unilateral breast reconstruction but also in bilateral, bipedicled and stacked breast reconstruction cases. Couplers demonstrated over 12 minutes time saving in bilateral reconstructions, 14 minutes in bipedicled and 10 minutes in stacked flaps. There were no coupler failures in any of the bilateral, bipedicled or stacked flaps. In these operations which add an extra layer of complexity and time as compared to unilateral reconstructions, the added benefit of time saved and potential reduced risk of thrombosis are significant factors which may well influence a surgeon’s decision to use couplers. The coupler is also recognised to reduce surgeon fatigue although this is difficult to quantify. In complex reconstructions the use of a coupler can dramatically reduce the complexity of required microsurgical demands. Multiple couplers can be used to extend vein grafts, in one case we have used five couplers in series to extend a vein graft.
Anastomosis site was a different variable we chose to explore. The use of a coupler was associated with a significant reduction in anastomosis time in both sites—the internal mammary system and the subscapular/thoracodorsal system. The time saving was greater using the coupler with the internal mammary artery and its perforators. It was also associated with a significant reduction in thrombosis rate at this site. When looking at the axilla there was a significant time saving but no difference in thrombosis rate. The coupler conveys time saving benefit irrespective of location of use, however in this cohort the thrombosis risk reduction benefit is only seen in its use on the anterior chest anastomosis location.
The benefits to microvascular coupler anastomosis can be seen however there are some documented drawbacks. There is a learning curve to correct use of the system which surgeons used to hand sewn anastomosis will need to adjust to. There is the theoretical deskilling of the ability to perform a robust hand sewn venous anastomosis, although until the advent of a reliable arterial coupler this skill will always be used (3). Cost issues have previously been cited as a disadvantage to the use of the coupler system however our data suggests that at worst it’s a cost neutral device and at best it can save a substantial cost in the prevention of reoperation and flap failure.
Evolution in the coupler devices have continued over time, with a broader range of sizes available, improvements in the instrumentation for applying the vessels to the coupler device, and even couplers with in-built implantable Doppler probes for monitoring. With further technological advance, outcome measures may improve on current rates even further.
Conclusions
The anastomotic coupler for venous anastomosis in free flap surgery is associated with reduced operating times, reduced take-backs to theatre and cost benefits. This is the first study to demonstrate clear clinical benefits to anastomotic couplers, and suggests that these may be the gold standard for venous microanastomosis. With increasing experience with their use and technological advances, these outcomes may continue to improve.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nakayama K, Tamiya T, Yamamoto K, et al. A simple new apparatus for small vessel anastomosisi (free autograft of the sigmoid included). Surgery 1962;52:918-31. [PubMed]
- Ostrup LT, Berggren A. The UNILINK instrument system for fast and safe microvascular anastomosis. Ann Plast Surg 1986;17:521-5. [PubMed]
- Ardehali B, Morritt AN, Jain A. Systematic review: anastomotic microvascular device. J Plast Reconstr Aesthet Surg 2014;67:752-5. [PubMed]
- Galanis C, Nguyen P, Koh J, et al. Microvascular lifeboats: a stepwise approach to intraoperative venous congestion in DIEP flap breast reconstruction. Plast Reconstr Surg 2014;134:20-7. [PubMed]
- Nahabedian MY, Momen B, Manson PN. Factors associated with anastomotic failure after microvascular reconstruction of the breast. Plast Reconstr Surg 2004;114:74-82. [PubMed]
- Wain RA, Whitty JP, Dalal MD, et al. Blood flow through sutured and coupled microvascular anastomoses: a comparative computational study. J Plast Reconstr Aesthet Surg 2014;67:951-9. [PubMed]
- Rozen WM, Whitaker IS, Acosta R. Venous coupler for free-flap anastomosis: outcomes of 1,000 cases. Anticancer Res 2010;30:1293-4. [PubMed]
- Lymperopoulos NS, Sofos S, Constantinides J, et al. Blood loss and transfusion rates in DIEP flap breast reconstruction. Introducing a new predictor. J Plast Reconstr Aesthet Surg 2013;66:1659-64. [PubMed]
- Paget JT, Young KC, Wilson SM. Accurately costing unilateral delayed DIEP flap breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:926-30. [PubMed]