
Narrative review of liver mobilization, diaphragm peritonectomy, full-thickness diaphragm resection, and reconstruction
Introduction
In 2018, approximately 22,240 patients were newly diagnosed with ovarian cancer (OC) and 14,070 patients died in the same year in the United States (1). In Korea, the incidence of OC has gradually increased in recent years (2), and the mortality rate of OC is higher than that of any other type of gynecologic cancer, thereby making OC the most lethal type. Standard treatment usually comprises primary cytoreductive surgery followed by adjuvant chemotherapy after diagnosis. However, two large randomized phase III clinical trials demonstrated the non-inferiority of neoadjuvant chemotherapy followed by interval cytoreductive surgery (3,4).
The importance of surgery in treating OC has been emphasized in several previous studies. Based on results of previous meta-analyses, maximum cytoreductive surgery is the most powerful prognostic clinical factor (5,6). In fact, many studies have shown that residual tumor size can significantly affect prognosis (7,8). Therefore, to achieve maximum cytoreduction during surgery, it is essential to perform various procedures on different organs. Tumor spread in the intraperitoneal region and lymph node involvement are the most common forms of OC metastases. The major sites of metastasis are gravity-dependent sites, such as the rectouterine pouch, bilateral paracolic gutters, ileocecal area, right diaphragmatic space, and rectosigmoid junctions (9-12).
With en bloc resection of the uterus, adnexa, and rectum, a metastatic mass in the pelvic area can be entirely removed (13). However, in the upper abdomen, it is impossible to perform en bloc resection of masses surrounding the spleen, pancreas, porta hepatis, liver, and lymph nodes around the lesser omentum and superior mesenteric artery because of the delicate and complex nature of the major organs nearby (14-17). Therefore, assessing the resectability of metastatic masses in the upper abdomen before surgery is important. Such masses are often also present in the diaphragm, and this study examines the procedure of resecting them. We reviewed and discussed the procedure of a cytoreductive surgery, including the resection of a tumor located in the upper abdomen together with that of a diaphragmatic mass. Additionally, postoperative care and possible complications were reviewed. We present the following article in accordance with the Narrative review reporting checklist (available at http://dx.doi.org/10.21037/gs-20-422).
Methods
For the purpose of this review, a literature search was conducted on the topics involving upper abdominal surgery in cases of epithelial OC in the PubMed and MEDLINE databases. Two authors (W Shin and J Mun) independently screened the titles and abstracts of the studies obtained in this search that was obtained using a combination of the following keywords: “ovarian neoplasm”, “ovarian cancer”, “ovarian malignancy”, “peritonectomy”, “upper abdominal surgery”, and “cytoreductive surgery”. We reviewed case reports, cross-sectional studies, and surgical videos pertaining to this topic. Cancers other than epithelial OC, such as colorectal cancer, gastric cancer, and pseudomyxoma with appendiceal cancer were excluded.
Results
Liver mobilization
Before diaphragmatic peritonectomy or full-thickness resection, the liver should be mobilized to visualize the entire diaphragm; however, before liver mobilization, understating the chief vasculature and attachment of the liver to its surrounding organs is important. Following the central line of the liver dome, the ligamentum teres attaches the liver to the upper abdominal wall. The anterior part of the ligamentum teres is connected to the falciform ligament, which superiorly continues while maintaining its connection to the anterior right and left coronary ligaments. These ligaments eventually continue to both lateral walls of the diaphragm and together form a triangular ligament together. In contrast, the round ligament exists along the posterior falciform ligament. The round ligament comprises the remnant umbilical vein and bile system; hence, it is important to be cautious when dissecting the round ligament from the liver because approaching too deeply can be dangerous.
Finally, the inferior vena cava (IVC) passes under the right side of the falciform ligament with bilateral hepatic veins draining into the anterior surface of the IVC at the level of the falciform peritoneal surface.
The order in which mobilization is performed is not significant. Several surgical recordings showing the common methods of liver mobilization are available (18-20). First, an incision is made from the pubis to the xiphoid process. A Balfour retractor is used to widen the incision on both sides, and a Kent retractor is placed on both sides of the diaphragm to ensure that there is no compromise on visibility and space. Similar retractors such as Omni or Bookwalter may also be utilized. Next, the falciform ligament is grasped using a Kelly clamp and then ligated, after which the surgeon follows the plane of the coronary ligament. Subsequently, the liver is slightly moved to the right with a malleable retractor to dissect the coronary ligament, thereby separating the diaphragm from the liver. On reaching the bare area, full mobilization is achieved (21). Although a monopolar coagulator device allows easy access to the area, it is necessary to pay attention to the IVC on the side of the liver dome. Additionally, if the liver is retracted to the left side of the patient during mobilization, the IVC may be compressed, thus possibly leading to a decrease in blood pressure. In such a case, it would be helpful to place the liver in a neutral position and wait for perfusion to return. When the liver is placed in a neutral position, blood pressure often recovers in less than 1 min, posing a low risk to the patient. A decrease in blood pressure can be avoided by elevating the liver to the left during retraction.
Once complete mobilization is achieved, a triangular ligament connected to the lateral wall of the diaphragm is visible, and this structure must be gently dissected. Next, the peritoneum of Morison’s pouch is dissected after the liver at the bottom portion is freed. The kidney is present under the lower retroperitoneum, which is why an assistant must gently push the kidney down further away from the liver, while another assistant uses a malleable retractor to hold the liver and gallbladder away from shadowing the retroperitoneum during dissection. If tumor seeding occurs in the upper part of the kidney, special attention must be paid to the adrenal gland in the retroperitoneal area to prevent injury. In the case of serious trauma to the adrenal gland during surgical resection, the possibility of the patient developing Addison’s disease and its treatment should be considered during postoperative management.
Diaphragm peritonectomy
The major components of the diaphragm are the peritoneum, muscle, and pleural membranes, the muscles of which are connected by tendons to the vertebra. In most cases, metastatic masses of OC that have shallowly invaded the diaphragm can be surgically stripped or resected. Metastases are usually identified on the right diaphragm by following the pattern of respiration and colonic peristalsis. If such a mass is also visible on the left diaphragm, peritonectomy with minimal liver mobilization can be performed. However, access to the posterior portion of the spleen is not easy, and splenectomy may be performed.
To perform diaphragm peritonectomy, the liver must be placed medially after complete liver mobilization. Next, using Mixter forceps, the peritoneum is incised with a monopolar coagulator device. The muscular area is then pushed toward the pleura using sponge forceps or surgical gauze. This portion can be easily removed provided there is no muscle invasion. Bleeding may occur and usually involves the muscle and can be controlled by bipolar devices, compression, or sutures. Other causes of bleeding may include damage to the vein present directly below the membrane of the diaphragmatic peritoneum (Figure 1). Full-thickness membrane resection may be necessary if muscle invasion is noted or if invasion up to the pleural membrane has been confirmed in imaging studies before surgery. Several such surgical video recordings have been published and they aid in providing an understanding of the surgical procedures involved (18-20,22,23).
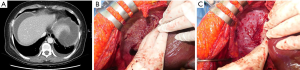
Full-thickness diaphragm resection and reconstruction
Diaphragm resection is required when muscles or pleural membranes are invaded. The affected area can be cut out in full thickness with a monopolar device, after which simple continuous suturing using either prolene 1-0 or vicryl 1-0 can be performed. Before closing the resected diaphragm, negative pressure must be applied to the pleural cavity using vacuum suction. The last tie must be placed after the air inside the pleural cavity is completely drawn out. Chest drains are often required at this time, either through the chest wall using a Jackson-Pratt (JP) catheter or through the diaphragm to drain via the abdominal cavity. If the ablation site is large (usually 20 cm2 or more), Gore-Tex may be required.
Right and left diaphragm
To access the right diaphragm, liver mobilization is necessary, and the process has been examined in the steps discussed above. In contrast, the left diaphragm is relatively easy to access because the liver does not block its visualization. During surgery, the left diaphragm is observed after omentectomy is performed in the spleen area. It is necessary to be careful not to tear the spleen owing to excessive retraction. The left diaphragm showed a relatively small tumor burden compared with the right diaphragm, and this may be because the settlement of tumor cells on the left side is unlikely to occur when considering the path of fluid flow in the abdominal cavity (24). During peritonectomy of the left diaphragm, surgeons need to be aware that there is no protective organ physically as is the case on the right side; therefore, they must exercise caution because muscle damage during peritonectomy may lead to diaphragmatic hernia (25).
Discussion
Peritonectomy vs. full-thickness diaphragm resection
If full-thickness invasion has not been confirmed, it is not necessary to perform full-thickness diaphragm resection. However, if the invasion has been established during peritonectomy and all other masses in the abdominal cavity have been removed along with the invasion site, full-thickness resection of the diaphragm becomes a reasonable option (14,26-31).
Complications
It is advisable to perform a bubble test after removing the diaphragmatic peritoneal membrane. Large perforations that occur during peritoneal dissection are easy to identify and repair; however, it is difficult to detect micro-perforations or defects that occur along the direction of the muscle (26).
Another complication is that OC is often accompanied by ascites; this fluid may enter the pleural space through the diaphragmatic region where the peritoneum is absent, resulting in pleural effusion (26). These complications may be avoided by inserting a prophylactic chest tube or JP catheter. Complications related to diaphragmatic peritonectomy or resections are summarized in Table 1.
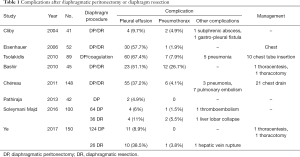
Full table
Postoperative care
It is recommended to perform chest radiography after the patient is moved to the recovery room (31). The patient’s vital signs, lung infiltration, and effusion must be monitored, and oxygen should be provided if necessary. Chest tube insertion during surgery can reduce the likelihood of postoperative complications.
Conclusions
The importance of maximal cytoreductive surgery in the treatment of OC is well known. In the field of gynecologic oncology, directing the attention of surgeons to the upper abdominal area and implementing active treatment plans will result in better outcomes for patients. Furthermore, the risk associated with diaphragm surgery is less compared with surgery involving other upper abdominal areas.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Gland Surgery for the series “Ultra-Radical Surgery in Ovarian Cancer: Surgical Techniques for Gynecologic Oncologist”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative review reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-422
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-422). The series “Ultra-Radical Surgery in Ovarian Cancer: Surgical Techniques for Gynecologic Oncologist” was commissioned by the editorial office without any funding or sponsorship. SYP served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284-96. [Crossref] [PubMed]
- Lim MC, Won YJ, Ko MJ, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999-2015. J Gynecol Oncol 2019;30:e38 [Crossref] [PubMed]
- Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943-53. [Crossref] [PubMed]
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386:249-57. [Crossref] [PubMed]
- Chang SJ, Bristow RE, Chi DS, et al. Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol 2015;26:336-42. [Crossref] [PubMed]
- Chang SJ, Bristow RE, Ryu HS. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann Surg Oncol 2012;19:4059-67. [Crossref] [PubMed]
- Chang SJ, Hodeib M, Chang J, et al. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol 2013;130:493-8. [Crossref] [PubMed]
- Selcuk I, Meydanli MM, Yalcin I, et al. Comparison of survival outcomes in optimally and maximally cytoreduced stage IIIC ovarian high-grade serous carcinoma: Women with only peritoneal tumor burden versus women with both peritoneal and lymphogenous dissemination. J Obstet Gynaecol Res 2019;45:2074-81. [Crossref] [PubMed]
- Meyers MA. Distribution of intra-abdominal malignant seeding: dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med 1973;119:198-206. [Crossref] [PubMed]
- Feki A, Berardi P, Bellingan G, et al. Dissemination of intraperitoneal ovarian cancer: Discussion of mechanisms and demonstration of lymphatic spreading in ovarian cancer model. Crit Rev Oncol Hematol 2009;72:1-9. [Crossref] [PubMed]
- Nougaret S, Addley HC, Colombo PE, et al. Ovarian carcinomatosis: how the radiologist can help plan the surgical approach. Radiographics 2012;32:1775-800; discussion 800-3. [Crossref] [PubMed]
- Yeung TL, Leung CS, Yip KP, et al. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol 2015;309:C444-56. [Crossref] [PubMed]
- Bristow RE, del Carmen MG, Kaufman HS, et al. Radical oophorectomy with primary stapled colorectal anastomosis for resection of locally advanced epithelial ovarian cancer. J Am Coll Surg 2003;197:565-74. [Crossref] [PubMed]
- Cliby W, Dowdy S, Feitoza SS, et al. Diaphragm resection for ovarian cancer: technique and short-term complications. Gynecol Oncol 2004;94:655-60. [Crossref] [PubMed]
- Magtibay PM, Adams PB, Silverman MB, et al. Splenectomy as part of cytoreductive surgery in ovarian cancer. Gynecol Oncol 2006;102:369-74. [Crossref] [PubMed]
- Manci N, Bellati F, Muzii L, et al. Splenectomy during secondary cytoreduction for ovarian cancer disease recurrence: surgical and survival data. Ann Surg Oncol 2006;13:1717-23. [Crossref] [PubMed]
- Zivanovic O, Eisenhauer EL, Zhou Q, et al. The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol 2008;108:287-92. [Crossref] [PubMed]
- Cordeiro Vidal G, Babin G, Querleu D, et al. Primary debulking surgery of the upper abdomen and the diaphragm, with a plasma device surgery system, for advanced ovarian cancer. Gynecol Oncol 2017;144:223-4. [Crossref] [PubMed]
- Lago V, Domingo S, Matute L, et al. Radical en bloc peritonectomy in advanced ovarian cancer. Ecancermedicalscience 2018;12:808. [Crossref] [PubMed]
- Nishikimi K, Tate S, Matsuoka A, et al. Resection of a metastatic bulky subphrenic tumor for the treatment of advanced ovarian cancer using liver mobilization and the Pringle maneuver. Gynecol Oncol 2018;151:176-7. [Crossref] [PubMed]
- Abdel-Misih SR, Bloomston M. Liver anatomy. Surg Clin North Am 2010;90:643-53. [Crossref] [PubMed]
- Sawyer BT, LaFargue CJ, Bristow RE. Extended left upper quadrant resection during primary cytoreductive surgery for Stage IV ovarian cancer. Gynecol Oncol 2016;142:378. [Crossref] [PubMed]
- Kato K, Katsuda T, Takeshima N. Cytoreduction of diaphragmatic metastasis from ovarian cancer with involvement of the liver using a ventral liver mobilization technique. Gynecol Oncol 2016;140:577-9. [Crossref] [PubMed]
- Torres D, Kumar A, Wallace SK, et al. Intraperitoneal disease dissemination patterns are associated with residual disease, extent of surgery, and molecular subtypes in advanced ovarian cancer. Gynecol Oncol 2017;147:503-8. [Crossref] [PubMed]
- Sakaguchi H, Masuda K, Kobayashi M, et al. Left Diaphragmatic Hernia After Diaphragmatic Peritonectomy for Peritoneal Cancer. J Clin Gynecol Obstet 2020;9:108-11. [Crossref]
- Eisenhauer EL, D'Angelica MI, Abu-Rustum NR, et al. Incidence and management of pleural effusions after diaphragm peritonectomy or resection for advanced mullerian cancer. Gynecol Oncol 2006;103:871-7. [Crossref] [PubMed]
- Bashir S, Gerardi MA, Giuntoli RL 2nd, et al. Surgical technique of diaphragm full-thickness resection and trans-diaphragmatic decompression of pneumothorax during cytoreductive surgery for ovarian cancer. Gynecol Oncol 2010;119:255-8. [Crossref] [PubMed]
- Chéreau E, Rouzier R, Gouy S, et al. Morbidity of diaphragmatic surgery for advanced ovarian cancer: retrospective study of 148 cases. Eur J Surg Oncol 2011;37:175-80. [Crossref] [PubMed]
- Pathiraja PN, Garruto-Campanile R, Tozzi R. Diaphragmatic peritonectomy versus full thickness diaphragmatic resection and pleurectomy during cytoreduction in patients with ovarian cancer. Int J Surg Oncol 2013;2013:876150 [Crossref] [PubMed]
- Soleymani Majd H, Ferrari F, Manek S, et al. Diaphragmatic peritonectomy vs. full thickness resection with pleurectomy during Visceral-Peritoneal Debulking (VPD) in 100 consecutive patients with stage IIIC-IV ovarian cancer: A surgical-histological analysis. Gynecol Oncol 2016;140:430-5. [Crossref] [PubMed]
- Ye S, He T, Liang S, et al. Diaphragmatic Surgery and Related Complications In Primary Cytoreduction for Advanced Ovarian, Tubal, and Peritoneal Carcinoma. BMC Cancer 2017;17:317. [Crossref] [PubMed]


