The biplanar oncoplastic technique case series: a 2-year review
Introduction
Breast conservation therapy (BCT) has evolved with the intent of removing a localized breast cancer while preserving the natural contour of the breast. The oncologic safety of this procedure has been well demonstrated and documented in numerous clinical studies with follow-up that now exceeds 20 years (1,2). Oncoplastic surgery has evolved with the intent of removing larger segments of the breast in order to ensure clear margins in patients where a lumpectomy may not be feasible (3,4). Various oncoplastic techniques have evolved in order to minimize or complete eliminate any contour deformity that may occur with such resections (5-8). The common reconstructive options for oncoplasty include volume displacement and volume replacement procedures. Volume displacement techniques include reduction mammaplasty, mastopexy, and glandular rearrangement and are typically reserved for women with larger breast volumes. Volume replacement techniques include the use of local or remote flaps that are typically used for women that are not candidates for volume displacement because of smaller breast volumes.
The biplanar technique was recently described as an option for women with small to moderate breast volume that were not candidates for a single modality of volume displacement, lacked sufficient remote tissue, or did not desire autologous volume replacement, and who did not want to have a mastectomy (9). The biplanar technique is a simultaneous combination of volume displacement and volume replacement method utilizing techniques of tissue rearrangement and device reconstruction. Although several studies have previously reported poor outcomes in the setting of breast radiation and delayed implant reconstruction, this technique differs in that the device is placed before radiation for the purpose of partial breast reconstruction (10,11).
The purpose of this study is to review our 2-year outcomes using this technique. Factors for review include patient selection, surgical technique, complications, and outcomes.
Methods
An IRB-approved retrospective review of patients who underwent oncoplastic surgery by the senior authors (RM and MYN) from 2011-2012 was performed. All patients that had the biplanar approach were included in the review. Patient demographics and perioperative details are included in Table 1. Patient selection criteria was based on the criteria mentioned previously: women with small to moderate breast volume that were not candidates for a single modality of volume displacement, lacked sufficient remote tissue, or did not desire autologous volume replacement, and who did not want to have a mastectomy.
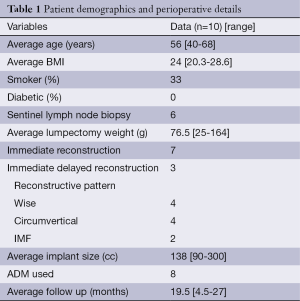
Full table
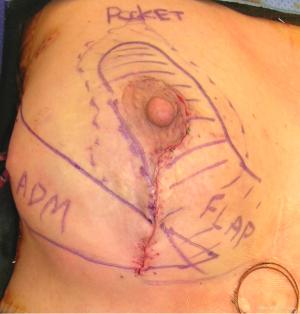
The biplanar technique has been previously described. The basic principles of this technique include simultaneous volume displacement and replacement using tissue rearrangement and devices, respectively. The tissue rearrangement was always in the form of a mastopexy. The incision pattern was circumvertical in four cases, a wise pattern in four cases and an inframammary fold incision in two cases. The partial mastectomy was performed by the ablative surgeon using the delineated pattern. Patients were given the option for immediate reconstruction based on intraoperative frozen section pathology or staged immediate reconstruction as defined as reconstruction prior to radiation, but after final pathology assessments. The skin flaps were elevated and the glandular resection completed. The reconstructive surgeon then performed the glandular rearrangement paying strict attention to the vascular anatomy in order to prevent devascularization of the remaining parenchyma and the nipple areolar complex. The surgical plan was to use inferior or lateral breast tissue to reconstruct the partial mastectomy defects. The subpectoral plane was entered and either a permanent silicone cohesive gel implant or a tissue expander was inserted. Acellular dermal matrix was used to support the lower pole tissues. A closed suction drain was inserted in all patients (Figure 1).
Results
Ten patients met the study criteria. The average patient age was 56 years (range, 40-68 years) and average BMI was 24.1 kg/m2 (range, 20.3-28.6 kg/m2) respectively. Average ablative resection weight was 76.5 grams (range, 25-164 g). The average ablative specimen volume was 95 cm3 (range, 35-411 cm3). Three patients had a final pathology of ductal carcinoma in situ, one had a pathology of ductal carcinoma in situ and invasive ductal adenocarcinoma, one patient had a pathology of ductal carcinoma in situ and lobular carcinoma in situ, three patients had pathology of invasive ductal adenocarcinoma, one patient had a pathology of invasive ductal adenocarcinoma and lobular carcinoma in situ and one patient had a pathology of invasive ductal adenocarcinoma with pleomorphic lobular carcinoma. A permanent implant was used in eight patients and a tissue expander was used in two patients. Acellular dermal matrix was used in nine patients. Immediate reconstruction was performed in seven patients, and three patients were reconstructed using the staged-immediate protocol to ensure clear tumor margins. Ablative resection site was in the upper outer quadrant in two patients, upper inner quadrant in three patients, lower outer quadrant in two patients and central location in three patients. Location of the pedicle for glandular rearrangement was lateral in two patients, superior in two patients, medial in four patients, inferior in one patient and central in one patient. Nine of the ten patients underwent radiation treatment, one patient had her radiation performed at a location outside of the author’s home institution and her records were unobtainable. The average days of radiation treatment was 32 days (range, 22-45 days), and the average number of factions was 22 (range, 16-28). All radiation was administered with tangential fields with a boost to the affected breast. The average total dosage was 5,563 cGy (range, 4,770-6,200 cGy) with the average boost of 1,193 cGy (range, 530-1,800 cGy).
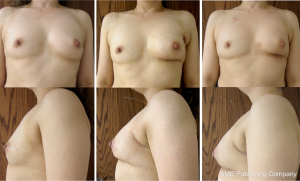
Following the glandular rearrangement, an implant was placed in the subpectoral space to replace the volume displaced from lateral and inferior quadrants. Average implanted volume was 138 cc (range, 90-300 cc). In general, ADM was used when the devices volume exceeded 125 cc and was not used when less than 125 cc. Tissue expanders were used in cases where the patient desired to have slightly larger breasts postoperatively (n=2). Both of these patients underwent contralateral augmentation postoperatively. Nipple sensation was maintained in 9 of 10 patients (complete loss of sensation was reported by one patient who underwent subsequent a mastectomy for positive margins). Follow-up ranged from 4.5-27 months (mean of 19.5 months) (Figure 2).
Complications were infrequent following this procedure. One patient developed a post-operative infection, prior to radiation, requiring explantation and a subsequent latissimus dorsi flap (prior staged immediate reconstruction). One patient developed a late complication occurred related to radiation-induced wound dehiscence resulting in implant exchange. One patient, who underwent an immediate reconstruction, had a positive margin requiring a completion mastectomy.
A basic satisfaction survey was conducted of all the patients. Five questions on the survey inquired about overall satisfaction, likelihood of doing the surgery again, recommending the procedure, perceived symmetry, and desiring further surgery. The responses were graded from 1 (least) to 5 (most). The results are stated in the Table 2.
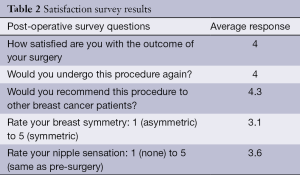
Full table
Discussion
Breast preserving procedures have become mainstay surgery for many women seeking therapeutic oncologic management. It has been estimated that 70% of patients diagnosed with breast cancer will be candidates for some type of BCT. The advantages of a partial mastectomy or quadrantectomy (>2 cm margin) over lumpectomy (<1 cm margin) are well understood (4). The benefits of immediate or staged immediate reconstruction are well appreciated and designed to be completed prior to the initiation of radiation therapy (12). Thus, the challenge to obtain reasonable cosmetic outcomes has been achieved with the various oncoplastic techniques are our disposal.
The experience with immediate oncoplastic breast surgery has been universally demonstrated to be safe and effective in properly selected patients (12-14). The benefits of performing an immediate contralateral symmetry procedure (when advisable) has also been evaluated (15). This has been effectively performed when placing permanent implants in the ipsilateral breast undergoing the oncoplastic procedure. A contralateral implant can be placed immediately based on the preoperative symmetry measurements, the volume of resected tissue on the opposite side, and the volume of the permanent implant on the opposite side.
The biplanar technique for oncoplastic reconstruction is relatively new and as such is not described in any textbook on this subject. Historically speaking, most attempts at volume restoration with implants were described using devices following radiation. This resulted in an unacceptable rate of capsular contracture, asymmetry, and other adverse events (16,17).
Partial breast reconstruction with prosthetic devices has been addressed either directly or indirectly in a number of studies. Petit et al. (18) looked at 111 cases of BCT and immediate reconstruction performed at institute of oncology in Milan. These included 11% that underwent immediate implant reconstruction. This technique resulted in a good result in 58% but with a complication rate of 75%. Mean follow-up was 21 months. The implants were used in larger reconstructions where local tissue use would not have been adequate. The location of the implant, either subpectoral or subcutaneous was not mentioned, nor if there were any other complementary reconstructive procedures performed.
Schaverien et al. (19) reviewed their experience with 23 delayed subglandular implant reconstruction after completions of BCT. Radiotherapy to implant time ranged 7-150 months. Follow-up after implant reconstruction was 8-101 months. They reported high satisfaction rates of all respondents to their questionnaire (all above an 8 out of 10 point scale). It is unclear what immediate local measures were taken to address the partial mastectomy defect, if any. They did state that four patients underwent a “mini latissimus dorsi” flap reconstruction.
Rietjens et al. (20) reported a series of patients having immediate reconstruction with placement of a subpectoral breast implant, glandular reapproximation, and intraoperative radiation (IORT). They report good outcomes at 1-year follow-up from an oncologic as well as an aesthetic standpoint. They also report that a prospective study evaluating BCT patients comparing IORT and conventional radiotherapy is underway. This study will also look at long term outcome immediate reconstruction after IORT.
Thomas et al. (8) reported on 59 patients who underwent a partial mastectomy and immediate placement of an implant in the lumpectomy pocket. Radiotherapy was performed in 64% of the patients. The explantation rate was 18.6% (11/59). Baker grade III/IV contractures were noted in 48%. Of those surveyed, 58% expressed satisfactory results.
The evolution of the biplanar technique was based on the concepts of breast conservation and avoidance of mastectomy in women with localized breast cancer who had small to moderate breast volumes. Traditional oncoplastic techniques for women with small to moderate breast volume were to use a local flap such as a latissimus dorsi or a thoracodorsal artery perforator flap (TDAP). However, some women lack sufficient tissue or do not want any additional scars so an alternative other than mastectomy was needed. The biplanar technique provides this option for women in this category. The concept behind this surgical plan was to reconstruct the partial mastectomy pocket with local breast tissue rearrangement techniques and then to augment the inferior or inferolateral pole (the more common sites of glandular tissue donor site) with the subpectoral implant. The placement of a prosthetic device in this setting is similar to that of a total mastectomy defect that undergoes reconstruction with immediate reconstruction. Patients with macromastia and severe micromastia are usually not candidates for this technique.
At the time of this preparation, the follow-up for these patients was less than 27 months. The number of patients is small but this partly due to the fact that there are few women that meet the criteria for inclusion. Most women have the expectation that contralateral procedure will be avoided. Some surgeons may have issue with this procedure based on the historic data related to implants and radiation in the setting of BCT and the reality is that this procedure will only be indicated in a few patients. The main benefit is avoiding a mastectomy as well as placement of additional scars on the body. With our current understanding of radiation therapy and device based reconstruction, studies have demonstrated acceptable results as long as the device is placed prior to the radiation. Although capsular contracture is a known and persistent risk, most women will have an acceptable outcome. Patients who have undergone the biplanar oncoplasty reconstruction have similar satisfaction rates as our other oncoplasty patients. We are looking forward to obtaining a long term (5 and 10 years) follow-up to assess the oncologic safety as well as the aesthetic outcome of these patients.
Our study has several shortcomings. Although retrospective in nature, it still provides insight as to the benefits of this technique and provides the groundwork for future investigation. The method of assessing patient satisfaction was not validated; however, moving forward, more sophisticated methods using the Breast-Q or the SF-36 can be implemented. The number of patients in this series is low; however, as we continue to follow these patients and modify our techniques, more patients can be considered and results may be more predictable. The purpose and goal of this study was to establish the technical feasibility of this technique and provide 2-year follow-up that was accomplished.
A combined submuscular implant-tissue rearrangement reconstruction may represent a valuable option in properly selected patients considering oncoplastic breast surgery. In addition to minimizing the incidence of contour irregularities, volume restoration was successfully restored and sometimes enhanced using this technique. A detailed discussion of risk and benefits is a prerequisite prior to offering this option to certain patients. Based on our early experience, patient satisfaction is high and long-term evaluations will determine if sustainable reconstructive outcomes are possible.
Acknowledgements
Disclosure: Dr. Nahabedian is a consultant for LifeCell Corp, Branchburg, New Jersey and Sientra Corp, Santa Barbara, CA. The other authors declare no conflict of interest.
References
- Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995;333:1456-61. [PubMed]
- Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 2011;103:478-88. [PubMed]
- Hill-Kayser CE, Vachani C, Hampshire MK, et al. Cosmetic outcomes and complications reported by patients having undergone breast-conserving treatment. Int J Radiat Oncol Biol Phys 2012;83:839-44. [PubMed]
- Kaur N, Petit JY, Rietjens M, et al. Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol 2005;12:539-45. [PubMed]
- Berry MG, Fitoussi AD, Curnier A, et al. Oncoplastic breast surgery: a review and systematic approach. J Plast Reconstr Aesthet Surg 2010;63:1233-43. [PubMed]
- Fitoussi AD, Berry MG, Couturaud B, et al. Management of the post-breast-conserving therapy defect: extended follow-up and reclassification. Plast Reconstr Surg 2010;125:783-91. [PubMed]
- Clough KB, Thomas SS, Fitoussi AD, et al. Reconstruction after conservative treatment for breast cancer: cosmetic sequelae classification revisited. Plast Reconstr Surg 2004;114:1743-53. [PubMed]
- Thomas PR, Ford HT, Gazet JC. Use of silicone implants after wide local excision of the breast. Br J Surg 1993;80:868-70. [PubMed]
- Nahabedian MY, Patel KM, Kaminsky AJ, et al. Biplanar oncoplastic surgery: a novel approach to breast conservation for small and medium sized breasts. Plast Reconstr Surg 2013;132:1081-4. [PubMed]
- Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg 2009;124:395-408. [PubMed]
- Jugenburg M, Disa JJ, Pusic AL, et al. Impact of radiotherapy on breast reconstruction. Clin Plast Surg 2007;34:29-37. [PubMed]
- Patel KM, Albino F, Fan KL, et al. Microvascular autologous breast reconstruction in the context of radiation therapy: comparing two reconstructive algorithms. Plast Reconstr Surg 2013;132:251-7. [PubMed]
- Rietjens M, Urban CA, Rey PC, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast 2007;16:387-95. [PubMed]
- Down SK, Jha PK, Burger A, et al. Oncological advantages of oncoplastic breast-conserving surgery in treatment of early breast cancer. Breast J 2013;19:56-63. [PubMed]
- Giacalone PL, Roger P, Dubon O, et al. Lumpectomy vs oncoplastic surgery for breast-conserving therapy of cancer. A prospective study about 99 patients. Ann Chir 2006;131:256-61. [PubMed]
- Spear SL, Willey SC, Robb GL, et al. editors. Surgery of the breast:principles and art. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2011.
- Nahabedian MY. editor. Oncoplastic Surgery of the Breast. St. Louis: Saunders Elsevier, 2009.
- Petit JY, Garusi C, Greuse M, et al. One hundred and eleven cases of breast conservation treatment with simultaneous reconstruction at the European Institute of Oncology (Milan). Tumori 2002;88:41-7. [PubMed]
- Schaverien MV, Stutchfield BM, Raine C, et al. Implant-based augmentation mammaplasty following breast conservation surgery. Ann Plast Surg 2012;69:240-3. [PubMed]
- Rietjens M, De Lorenzi F, Veronesi P, et al. Breast conservative treatment in association with implant augmentation and intraoperative radiotherapy. J Plast Reconstr Aesthet Surg 2006;59:532-5. [PubMed]


