Electrophysiological neural monitoring of the laryngeal nerves in thyroid surgery: review of the current literature
Introduction
Thyroid surgery is among the most common cervical surgeries. Due to the close proximity of the laryngeal nerves to the surgical field, nerve injury is a common complication of these surgeries. Unilateral paralysis of the recurrent laryngeal nerve (RLN) can cause dysphagia, hoarseness of voice, or respiratory complications due to aspiration (1). The consequences are more severe in bilateral paralysis, which can jeopardize the airway and may necessitate tracheostomy (1). As the external branch of the superior laryngeal nerve (EBSLN) supplies only the cricothyroid muscle, its paralysis is less devastating and can affect the voice variably.
RLN injury is the most common cause of medicolegal litigation after thyroid surgery (2). In cases of bilateral vocal cords paralysis, plaintiffs can be awarded up to 2.5 million dollars (1).
Rates of RLN injury are reported to be as high as 10% (3). Regarding the EBSLN, the reported injury rate varies widely (0-58%) due to difficulty of assessment (4). The published rates of RLN injury are thought to be underestimated, as the less satisfactory results are less commonly reported, and reported RLN injuries are usually from high-volume practices (5,6). Complications related to thyroid surgery, including laryngeal nerve palsy, are reported to be higher in low-volume surgeons (7,8).
Revision neck surgery may be especially challenging due to postoperative changes and scarring. The risk of RLN injury increases threefold for repeat thyroid surgery when compared to the initial operations (9).
Intraoperative visual identification has been the gold standard for securing the laryngeal nerves during thyroid surgery (10-12). However, an anatomically intact nerve identified by gross visualization does not confirm a functional nerve. Consequently, electrophysiologic intraoperative nerve monitoring (IONM) of the nerves was introduced by Shedd and Durham in 1965 as a novel technique which lowers the risk of RLN injury compared to the traditional visual identification (13).
IONM has been gaining popularity since its introduction. Studies have shown that over 50% of otolaryngologists and general surgeons use it in all or some of their cases (14,15). Neuromonitoring was reported to be more beneficial in video-assisted surgery as it makes the surgeon more comfortable during nerve dissection (5). Keeping detailed records of perioperative visits, including fundamental discussions, is essential. RLN injury should be emphasized as a potential complication and clear documentation in the informed consent is crucial. Nerve stimulating current, response amplitude and latency should be recorded in the operative report during and by the end of surgery.
The aim of our review is to review the literature addressing IONM to assess it safety, feasibility in thyroid surgery. Herein, we are also discussing anatomy of laryngeal nerves and aspects of its injury; and concise description of the basics of neuromonitoring.
Surgical anatomy of the laryngeal nerves
Recurrent laryngeal nerve (RLN)
The RLN is a branch of the vagus nerve. The right RLN originates at the level of the right subclavian artery and loops around it; then ascends in the tracheo-esophageal groove to the larynx. On the left side, it originates in the superior mediastinum at the level of the aortic arch, curves around it, runs superiorly through the thoracic outlet, and travels in the tracheo-esophageal groove near the branches of the inferior thyroid artery. It gives sensory branches to the esophagus and trachea before entering the larynx. Extralaryngeal branching of the RLN into anterior and posterior branches was reported to occur in around 40% of patients (16,17) (Figure 1). The motor fibers are usually located in the anterior branch; however they may run in the posterior branch that mostly carries sensory fibers (18). Extralaryngeal branching should be identified to avoid missing any unidentified anterior branch which may lead to postoperative nerve injury (16).
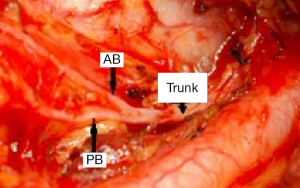
There are four important landmarks to aid mapping the RLN in thyroid surgery (2,19):
- The tracheo-esophageal groove, taking into consideration that the course of the RLN near or in the groove varies between individuals, especially on the right side;
- The inferior thyroid artery, another variable landmark of the RLN. The relationship of the nerve to the vessel is not constant and is a common site of RLN injury;
- The inferior thyroid cartilage cornu, which is palpable manually;
- The entry to the larynx, believed to be the most reliable RLN landmark and the most common site of injury. It is more vulnerable at this point due to close proximity of the nerve to Berry’s ligament and the thyroid capsule (2).
External branch of the superior laryngeal nerve (EBSLN)
The EBSLN is a branch of the superior laryngeal nerve, which originates at the C2 spinal level from the vagus nerve and divides into internal and external branches. It then descends behind the carotid sheath and the superior thyroid artery towards the superior pole of the thyroid. The nerve can be located at the laryngeal head of the sternothyroid muscle.
In cases with large goiters or upper pole masses, the upper edge of this muscle could be transected, applying traction to the upper thyroid pole and dissecting the sternohyoid-laryngeal triangle to facilitate EBSLN exposure. The EBSLN runs close to the superior thyroid vessels, a location which makes it vulnerable to injury during their ligation. Due to the variability of the EBSLN course, classifications were suggested to identify the relationship of the EBSLN to the upper thyroid pole and superior thyroid vessels (20,21). Cernea et al. classified EBSLN into type 1 which crosses the superior thyroid vessels more than 1 cm above the upper thyroid pole, type 2A that crosses the vessels less than 1 cm from the upper pole, and type 2B that crosses the vessels below the upper pole (20). In 68-90% of cases, the EBSLN was reported to share with the RLN in innervating the anterior thyroid muscle as the communicating branch (22).
Nerve injury
Laryngeal nerves are mainly injured by traction, suture entrapment, transection or thermal injury. Thermal injury is caused by energy-generating devices that can cause collateral damage to nearby structures. The International Neural Monitoring Study Group classified nerve injury into two categories: segmental, which involves a lesion to a clear-cut segment of the RLN, and global, where all the RLN and vagus are nonconductive which may indicate an intralaryngeal focus of injury (23). The segmental injury may be potentially correctable in case of a suture or clip entrapping the nerve that can be removed to prevent permanent nerve injury.
Revision neck surgery and surgery after radiotherapy have a higher risk of nerve injury due to tissue scarring. In large goiters, Graves’ disease and thyroiditis, enlarged glands put nerves under tension, making them more vulnerable to injury. The right non-recurrent inferior laryngeal nerve which is associated with arteria lusoria (aberrant right subclavian artery) carries a higher risk of injury due to its unusual course. Because dissection is more difficult in extralaryngeal branches of the RLN, they are at increased risk of injury compared to single nerves.
IONM technique (Figure 2)
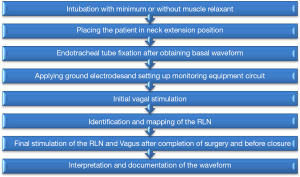
Cooperation between the surgeon and the anesthesiologist is essential for successful neuromonitoring. The use of neuromuscular blockers should be carefully considered and avoided if possible, as they reduce response amplitude from the vagus, RLN and EBSLN which may hinder injury detection.
In IONM, an endotracheal tube (ETT) tube equipped with a pair of recording electrodes of suitable size to make direct contact with the vocal cords is used. Due to its safety and simplicity, it is superior to other modalities as glottic observation, laryngeal palpation, postcricoid surface electrodes and intramuscular electrodes (23).
A stimulator probe is used to deliver the electrical current to structures suspected to be neural tissue. For identification and mapping of the RLN and EBSLN, a probe is used to deliver intermittent current when needed. A 2 mA current is used initially for nerve identification and mapping when overlaid by other tissue. A current of 1 mA can be used to map the nerve once identified and exposed. An automatic periodic stimulation (APS) probe is placed over the vagus nerve for real-time continuous monitoring. The nerve is exposed by dissecting the carotid sheath for 1 cm. It works by delivering a continuous low level stimulation to the vagus to obtain a basic nerve function. It gives a real time feedback by monitoring EMG responses. Continuous IONM (C-IONM) can detect early change in nerve function which may be a warning of impending nerve injury.
The stimulating and recording equipment are connected to an interface connector box, which is connected to grounding electrodes. Both the stimulatory and recording grounding electrodes are placed on the shoulder or on the sternum. The interface box is connected to a monitoring device. Waveform monitors are superior to audio systems with alarms indicating signal abnormality, as visual waveform displays amplitude, threshold and latency data which can differentiate true signals from artifacts.
Respiratory movement EMG waveform is used as proof of correct positioning and contact between recording electrodes and vocal cords. Baseline amplitude of 30-70 mV indicates proper positioning of the ETT (24). Proper positioning of the ETT can be verified by other methods as: fiberoptic laryngoscopic examination, tap test on the larynx or translaryngeal stimulation. The RLN is searched for in the tracheoesophageal groove near the inferior thyroid artery. To localize the EBSLN, the probe can be placed between the laryngeal head of the sternothyroid muscle and the superior thyroid pole (4,25). Visualization of the EBSLN is more challenging than RLN; in a previous study for our group the rate of visualization was about 53% (25). EBSLN visualization was more challenging in obese patients with two-fold chance to visualize it in non-obese patients (25). While RLN response is evaluated by the EMG waveform displayed on the monitor, EBSLN response is evaluated by monitoring cricothyroid muscle twitch.
Feasibility and safety of IONM and medico-legal implications
IONM is considered a feasible and safe addition to traditional visualization in identifying nerves (26,27). It was reported that IONM can reduce the rate of transient RLN injury in thyroid surgery (11,28,29). Interestingly, one study notes the conspicuous absence of IONM use in cases of bilateral RLN injury related to thyroid surgery (30). Another study has shown that users of IONM are less frequently named in lawsuits involving RLN injury, compared with non-users (14). The major motivations for neuromonitoring that surgeons cite are enhanced confidence during operative nerve dissection and diminished medicolegal liability (15).
However, there is no clear consensus in the literature to indicate that IONM decreases RLN injury. This is most probably due in part to the low incidence of injury in hands of high volume surgeons. Other studies showed that IONM did not reduce the prevalence of RLN palsy (31,32). Due to the low rate of RLN palsy, Dralle et al. reported that running a statistically powerful study would require millions of patients (11).
Recent guidelines from the American Academy of Otolaryngology-Head and Neck Surgery recommend IONM use in thyroid surgery to ensure voice protection (33). The German Association of Endocrine Surgeons practice guidelines and the International Neural Monitoring Study Group guidelines both support using IONM in all thyroid surgeries (23,34), while the American Head and Neck Society endorses its utilization in thyroid cancer cases, particularly patients with RLN palsy.
IONM was reported to decrease operative time when compared to visual identification alone as it decreases the time needed to identify the RLN (35). Final monitoring signal can prognosticate postoperative nerve function with high reliability. In the case of non-reduced signal, the negative predictive value of IONM can be as high as 97%, while the positive predictive value when signal is lost is only 33-37.8% (11,28).
Preoperative laryngoscopy is proposed to be significant in all patients when counseling and planning extent of surgery (36,37). However, it is believed by others to be more essential in patients with persistent hoarseness of voice, suspicious for cancer or with prior thyroid surgery (33,38). Detection of any preoperative baseline abnormality is essential, as preoperative paralysis of the vocal cords may be an indicator of locally advanced cancer (36). Nonetheless, Kamani et al. found that 60% of invaded nerves retained near-normal EMG activity amplitude and normal function of vocal cords preoperatively (39).
Routine postoperative laryngoscopy is recommended, as hoarseness does not necessarily indicate abnormal glottic function (36). Patients with intact nerves may experience hoarseness, while others with a paralyzed cord can be asymptomatic. Laryngoscopy is necessary to differentiate between vocal cord paralysis and intubation-induced causes of hoarseness such as vocal cord polyps.
IONM can determine the mechanism of injury by tracking surgical maneuvers after which the signal was lost. Compared to visual identification which detects only 10% of nerve injuries, IONM can predict postoperative vocal cord function (2). Postoperative hemi-laryngeal edema can cause vocal cord immobility with normal signal; once the edema subsides, mobility will be regained.
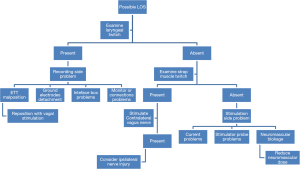
Initial amplitude of 100 mV is essential to ensure the system is functional and any subsequent loss of signal (LOS) is not due to equipment malfunction (Figure 3 shows stepwise approach for LOS). IONM can diagnose non-RLN which has shorter latency time compared to the recurrent nerve (11). Prolonged latency time can be used in combination with decreased amplitude or complete LOS as an indicator of nerve injury (23,40). Post-dissection amplitude off 200 µV was used as a cutoff to predict RLN injury and showed an accuracy of 99.1% in prognosticating postoperative RLN function (40).
IONM may help in avoiding bilateral RLN injury with its devastating effects on airway which may necessitate tracheostomy. In bilateral surgery, when nerve injury is detected by IONM on the initial side, the procedure could be staged by postponing operation on the other side until vocal cord function can be verified (41,42).
Repetitive nerve stimulation in IONM is safe and does not affect the functional integrity of nerves; there was no decline in amplitude after repetitive stimulation (1,43). Although a current of 1-2 mA is advisable to minimize the potential for false results of high current shunting and is enough for a maximum EMG response, a higher current can be tolerated (44).
Despite IONM is considered safe, it has some limitations. EMG signal can be affected by anesthesia and manipulation of the trachea. In addition, it is difficult to differentiate between nerve injury and loss of contact between the recording electrodes and the vocal cords in case of EMG amplitude or LOS. Intermittent IONM is limited to the short intervals of stimulation, so it usually detects nerve injury after it has occurred. Moreover, it can miss injury proximal to the point of stimulation as it only examines the nerve distal to the point of stimulation.
These limitations of intermittent IONM which can expose the laryngeal nerves to injury proximal to the site of stimulation or the interval gap between two intermittent stimulations are overcome by C-IONM (45). C-IONM is a modality of neuromonitoring that uses a stimulator clip applied to the vagus nerve and delivers interrupted periodic stimulation. It examines the whole course of RLN by continuous stimulation of the vagus nerve to detect proximal injuries that can be missed by intermittent stimulation distal to the injury. It gives feedback about nerve function at short intervals. If EMG signal decreased or became weak indicating imminent nerve injury, an adverse condition may be reversed before nerve damage is persistent. This is achievable in reversible nerve injury as neuropraxia, in contrast to significant disruption as complete transection of nerves (46). Furthermore, it can detect the most proximal RLN or EBSLN injuries (23). The vagus nerve has varied relation to the carotid artery and the internal jugular vein, so the electrode used in C-IONM should be flexible (46). The procedure was considered safe in a German study of 30 nerves at risk in 24 patients (47). Although continuous monitoring is an improvement in laryngeal nerves monitoring, an intermittent stimulator is still important in mapping the nerves in combination with continuous monitoring. In a prospective multicenter study that included 102 thyroidectomies; C-IONM showed safety and predictability of vocal cord palsy in case of LOS or combined event of decreased amplitude and increased latency (48). Combined events were found to be reversible when the surgical maneuver is stopped or modified saving the nerve from permanent injury (48). Other studies supported its accuracy and reliability in detecting impending nerve injury that can be reversed to save the nerve (49-51).
Conclusions
Although there is no consensus that IONM decreases laryngeal nerve injury when compared to visual identification, it has many advantages over traditional nerve visualization alone. IONM may help prevent bilateral RLN injury by allowing surgeons to stage the surgery when the signal is lost on the initial side, thus avoiding the need for tracheostomy. In addition, IONM can properly prognosticate postoperative nerve function, which is difficult to detect by visual identification, as most of injured nerves appear intact. Neuromonitoring can detect anatomical variation and abnormal courses of the nerves which are at higher risk of injury if not detected. Higher risk patients, including cancer patients, patients with prior surgery and scarring, and patients with large goiters, may benefit most from IONM. C-IONM is an advanced modality of neuromonitoring that can prevent potential nerve injury by detecting signal changes indicating an adverse condition, such as a suture compressing the nerve. C-IONM is also able to detect the most proximal injuries, which may be missed by intermittent monitoring. More large studies are needed to further clarify the benefits of these of these approaches.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Caragacianu D, Kamani D, Randolph GW. Intraoperative monitoring: normative range associated with normal postoperative glottic function. Laryngoscope 2013;123:3026-31. [PubMed]
- Durán Poveda MC, Dionigi G, Sitges-Serra A, et al. Intraoperative monitoring of the recurrent laryngeal nerve during thyroidectomy: A standardized approach (Part 1). World Journal of Endocrine Surgery 2011;3:144-50.
- Lo CY, Kwok KF, Yuen PW. A prospective evaluation of recurrent laryngeal nerve paralysis during thyroidectomy. Arch Surg 2000;135:204-7. [PubMed]
- Barczyński M, Randolph GW, Cernea CR, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013;123 Suppl 4:S1-14. [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Neuromonitoring and video-assisted thyroidectomy: a prospective, randomized case-control evaluation. Surg Endosc 2009;23:996-1003. [PubMed]
- Snyder SK, Lairmore TC, Hendricks JC, et al. Elucidating mechanisms of recurrent laryngeal nerve injury during thyroidectomy and parathyroidectomy. J Am Coll Surg 2008;206:123-30. [PubMed]
- Kandil E, Noureldine SI, Abbas A, et al. The impact of surgical volume on patient outcomes following thyroid surgery. Surgery 2013;154:1346-52. [PubMed]
- Loyo M, Tufano RP, Gourin CG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope 2013;123:2056-63. [PubMed]
- Kurmann A, Herden U, Schmid SW, et al. Morbidity rate of reoperation in thyroid surgery: a different point of view. Swiss Med Wkly 2012;142:w13643. [PubMed]
- Stevens K, Stojadinovic A, Helou LB, et al. The impact of recurrent laryngeal neuromonitoring on multi-dimensional voice outcomes following thyroid surgery. J Surg Oncol 2012;105:4-9. [PubMed]
- Dralle H, Sekulla C, Haerting J, et al. Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 2004;136:1310-22. [PubMed]
- Snyder SK, Hendricks JC. Intraoperative neurophysiology testing of the recurrent laryngeal nerve: plaudits and pitfalls. Surgery 2005;138:1183-91. [PubMed]
- Shedd DP, Durham C. Electrical identification of the recurrent laryngeal nerve. I. Response of the canine larynx to electrical stimulation of the recurrent laryngeal nerve. Ann Surg 1966;163:47-50. [PubMed]
- Sturgeon C, Sturgeon T, Angelos P. Neuromonitoring in thyroid surgery: attitudes, usage patterns, and predictors of use among endocrine surgeons. World J Surg 2009;33:417-25. [PubMed]
- Singer MC, Rosenfeld RM, Sundaram K. Laryngeal nerve monitoring: current utilization among head and neck surgeons. Otolaryngol Head Neck Surg 2012;146:895-9. [PubMed]
- Kandil E, Abdel Khalek M, Aslam R, et al. Recurrent laryngeal nerve: significance of the anterior extralaryngeal branch. Surgery 2011;149:820-4. [PubMed]
- Kandil E, Abdelghani S, Friedlander P, et al. Motor and sensory branching of the recurrent laryngeal nerve in thyroid surgery. Surgery 2011;150:1222-7. [PubMed]
- Fontenot TE, Randolph GW, Friedlander PL, et al. Gender, race, and electrophysiologic characteristics of the branched recurrent laryngeal nerve. Laryngoscope 2014;124:2433-7. [PubMed]
- Miller MC, Spiegel JR. Identification and monitoring of the recurrent laryngeal nerve during thyroidectomy. Surg Oncol Clin N Am 2008;17:121-44. viii-ix. [PubMed]
- Cernea CR, Ferraz AR, Furlani J, et al. Identification of the external branch of the superior laryngeal nerve during thyroidectomy. Am J Surg 1992;164:634-9. [PubMed]
- Selvan B, Babu S, Paul MJ, et al. Mapping the compound muscle action potentials of cricothyroid muscle using electromyography in thyroid operations: a novel method to clinically type the external branch of the superior laryngeal nerve. Ann Surg 2009;250:293-300. [PubMed]
- Sañudo JR, Maranillo E, León X, et al. An anatomical study of anastomoses between the laryngeal nerves. Laryngoscope 1999;109:983-7. [PubMed]
- Randolph GW, Dralle H, Abdullah H, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121 Suppl 1:S1-16. [PubMed]
- Durán Poveda MC, Dionigi G, Sitges-Serra A, et al. Intraoperative monitoring of the recurrent laryngeal nerve during thyroidectomy: A standardized approach part 2. World Journal of Endocrine Surgery 2012;4:33-40.
- Kandil E, Mohamed SE, Deniwar A, et al. Electrophysiologic identification and monitoring of the external branch of superior laryngeal nerve during thyroidectomy. Laryngoscope 2015. [Epub ahead of print]. [PubMed]
- Calò PG, Pisano G, Medas F, et al. Intraoperative recurrent laryngeal nerve monitoring in thyroid surgery: is it really useful? Clin Ter 2013;164:e193-8. [PubMed]
- Terris DJ, Anderson SK, Watts TL, et al. Laryngeal nerve monitoring and minimally invasive thyroid surgery: complementary technologies. Arch Otolaryngol Head Neck Surg 2007;133:1254-7. [PubMed]
- Barczyński M, Konturek A, Cichoń S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 2009;96:240-6. [PubMed]
- Cavicchi O, Caliceti U, Fernandez IJ, et al. The value of neurostimulation and intraoperative nerve monitoring of inferior laryngeal nerve in thyroid surgery. Otolaryngol Head Neck Surg 2009;140:866-70. [PubMed]
- Dralle H, Lorenz K, Machens A. Verdicts on malpractice claims after thyroid surgery: emerging trends and future directions. Head Neck 2012;34:1591-6. [PubMed]
- Shindo M, Chheda NN. Incidence of vocal cord paralysis with and without recurrent laryngeal nerve monitoring during thyroidectomy. Arch Otolaryngol Head Neck Surg 2007;133:481-5. [PubMed]
- Atallah I, Dupret A, Carpentier AS, et al. Role of intraoperative neuromonitoring of the recurrent laryngeal nerve in high-risk thyroid surgery. J Otolaryngol Head Neck Surg 2009;38:613-8. [PubMed]
- Chandrasekhar SS, Randolph GW, Seidman MD, et al. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg 2013;148:S1-37. [PubMed]
- Musholt TJ, Clerici T, Dralle H, et al. German Association of Endocrine Surgeons practice guidelines for the surgical treatment of benign thyroid disease. Langenbecks Arch Surg 2011;396:639-49. [PubMed]
- Sarı S, Erbil Y, Sümer A, et al. Evaluation of recurrent laryngeal nerve monitoring in thyroid surgery. Int J Surg 2010;8:474-8. [PubMed]
- Randolph GW, Kamani D. The importance of preoperative laryngoscopy in patients undergoing thyroidectomy: voice, vocal cord function, and the preoperative detection of invasive thyroid malignancy. Surgery 2006;139:357-62. [PubMed]
- Farrag TY, Samlan RA, Lin FR, et al. The utility of evaluating true vocal fold motion before thyroid surgery. Laryngoscope 2006;116:235-8. [PubMed]
- Schlosser K, Zeuner M, Wagner M, et al. Laryngoscopy in thyroid surgery--essential standard or unnecessary routine? Surgery 2007;142:858-64; discussion 864.e1-2.
- Kamani D, Darr EA, Randolph GW. Electrophysiologic monitoring characteristics of the recurrent laryngeal nerve preoperatively paralyzed or invaded with malignancy. Otolaryngol Head Neck Surg 2013;149:682-8. [PubMed]
- Genther DJ, Kandil EH, Noureldine SI, et al. Correlation of final evoked potential amplitudes on intraoperative electromyography of the recurrent laryngeal nerve with immediate postoperative vocal fold function after thyroid and parathyroid surgery. JAMA Otolaryngol Head Neck Surg 2014;140:124-8. [PubMed]
- Giordano D, Valcavi R, Thompson GB, et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 2012;22:911-7. [PubMed]
- Fontenot TE, Randolph GW, Setton TE, et al. Does intraoperative nerve monitoring reliably aid in staging of total thyroidectomies? Laryngoscope 2015. [Epub ahead of print]. [PubMed]
- White WM, Randolph GW, Hartnick CJ, et al. Recurrent laryngeal nerve monitoring during thyroidectomy and related cervical procedures in the pediatric population. Arch Otolaryngol Head Neck Surg 2009;135:88-94. [PubMed]
- Wu CW, Lu IC, Randolph GW, et al. Investigation of optimal intensity and safety of electrical nerve stimulation during intraoperative neuromonitoring of the recurrent laryngeal nerve: a prospective porcine model. Head Neck 2010;32:1295-301. [PubMed]
- Schneider R, Przybyl J, Hermann M, et al. A new anchor electrode design for continuous neuromonitoring of the recurrent laryngeal nerve by vagal nerve stimulations. Langenbecks Arch Surg 2009;394:903-10. [PubMed]
- Dionigi G, Donatini G, Boni L, et al. Continuous monitoring of the recurrent laryngeal nerve in thyroid surgery: a critical appraisal. Int J Surg 2013;11 Suppl 1:S44-6. [PubMed]
- Lamadé W, Ulmer C, Friedrich C, et al. Signal stability as key requirement for continuous intraoperative neuromonitoring. Chirurg 2011;82:913-20. [PubMed]
- Phelan E, Schneider R, Lorenz K, et al. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 2014;124:1498-505. [PubMed]
- Schneider R, Randolph GW, Sekulla C, et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013;35:1591-8. [PubMed]
- Schneider R, Bures C, Lorenz K, et al. Evolution of nerve injury with unexpected EMG signal recovery in thyroid surgery using continuous intraoperative neuromonitoring. World J Surg 2013;37:364-8. [PubMed]
- Van Slycke S, Gillardin JP, Brusselaers N, et al. Initial experience with S-shaped electrode for continuous vagal nerve stimulation in thyroid surgery. Langenbecks Arch Surg 2013;398:717-22. [PubMed]


