Current strategies with 1-stage prosthetic breast reconstruction
Introduction
Breast cancer is a leading cause of morbidity and mortality in women of all ages (1). An increasing number of these women and those who have the breast cancer gene are choosing unilateral or bilateral mastectomy to treat or prevent breast cancer (2). The etiology of this interesting trend is multifactorial, but it is likely influenced by improved techniques in mastectomy and reconstruction. The evolution of mastectomy to skin-sparing and nipple-sparing procedures has offered an opportunity to create natural breast reconstructions, and it has increased the number of patients eligible for 1-stage direct-to-implant (DTI) reconstruction (3). Our ability to obtain excellent cosmetic results with implants adds to the known advantages of implant-based reconstruction including a shorter operative time, lack of donor-site morbidity, and quicker return to normal life activities. This is turn has decreased the number of patients who now seek autologous reconstruction in the United States (4).
DTI breast reconstruction has appeal to patient and surgeon alike. For the patient, a DTI reconstruction allows the potential for completion of the entire reconstruction process in a single surgery, which avoids extra office visits and a second surgery to exchange the tissue expander to a permanent implant. However, not all patients are candidates for 1-stage prosthetic breast reconstruction. This article reviews patient selection, technical pearls, postoperative management, complications, and outcomes in DTI breast reconstruction.
Patient selection
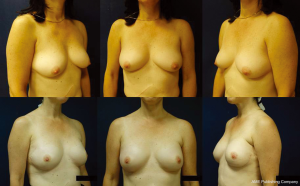
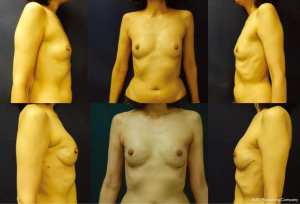
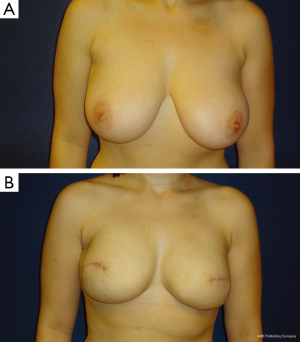
The ideal candidate for 1-stage breast reconstruction is a healthy patient who desires to stay about the same breast size (Figures 1,2). Nipple-sparing mastectomy procedures offer more skin to accommodate a full size implant; however, skin-sparing mastectomies offer more of an uplift for the woman with large breasts looking to decrease her size (Figure 3A,B). If the patient has significant co-morbidities such as uncontrolled diabetes, a history of transplantation, or active smoking, a 2-stage or delayed reconstruction is often advised. Advancing age, obesity, radiation, and prior breast surgery are not contraindications to 1-stage reconstruction, but patients need to be considered on an individual basis (3,5-7). With skin-sparing or skin-reducing mastectomies it is possible to make the patient much smaller in size in one surgery, whereas significant size reduction with nipple-sparing mastectomies is more challenging. If the mastectomy skin envelope is ideal, it is sometimes possible to make the patient larger in size than their natural breast. However, significant size enhancements are more safely done with a 2-stage tissue expander-implant reconstruction.
Technical pearls
Preoperative Planning: In the initial consultation, an assessment of patient size goals and native breast symmetry is noted. The breast diameter is measured and the volume approximated to ensure the right implants are available on the day of surgery. It is important to discuss asymmetries and plan for differences in inframammary fold location. A paravertebral block is given for perioperative pain control.
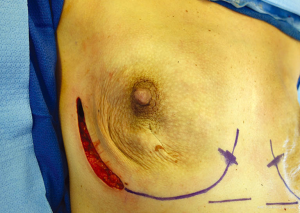
Marking: The inframammary folds and lateral borders of the breasts are marked with the patient in the sitting or standing position. Incision placement is determined with the breast oncologic surgeon. A decision is made on a skin-reducing, skin-sparing, or nipple-sparing approach. For nipple-sparing mastectomies, an inferolateral incision allows excellent access for mastectomy, lymph node sampling, and reconstruction (Figure 4) (8). However, a radial incision maximizes blood flow and should be considered when the blood supply to the nipple or skin may be compromised, such as in cases where thin mastectomy skin flaps are anticipated and in cases with scars on the breast.
Preparation: The skin is prepped with a betadine or chlorhexidine scrub. Once the mastectomies are finished, the skin is prepped once again and new sterile drapes are placed on top of the original drapes. The arms are angled approximately 75 degrees from the operating table on arm boards. A muscle relaxant is given to facilitate partial subpectoral implant placement.
Procedure: The pectoralis muscle is elevated from lateral to medial and the inferior attachment is released with electrocautery to approximately 4 or 8 o’clock. A piece of acellular dermal matrix (ADM) or mesh is sewn to the inframammary fold or chest wall inferiorly and to the chest wall (or serratus flap) laterally (Figure 5). Care is taken to leave some laxity medially to allow the implant to assume a medial position. Simple interrupted sutures are used inferiorly and horizontal mattress sutures are used laterally to the chest wall. Alternatively the ADM or mesh can first be sewn to the pectoralis muscle and the inferior/lateral suturing can then be performed after placement of the implant. A sizer is placed inside the newly created pocket and sewn in place. The patient is sat upright and the sizer is inflated to check for proper pocket placement and to determine size. An implant is chosen that best matches the width of the pocket and the volume determined by the sizer. Anatomic/shaped implants are often preferred for unilateral reconstructions and patients with thin skin. Round implants offer more upper pole volume and mobility making them more attractive to younger patients. Prior to implant placement, the pocket is irrigated with a triple antibiotic solution containing Cefazolin, Gentamycin, and Bacitracin. One drain is placed inside the pocket along the inframammary fold and another drain is placed laterally outside the pocket and travels over the superior surface of the muscle. Care is taken to make a separate stab incision for drain exit and to tunnel 1-2 cm within the tissue prior to exiting. The surgeon’s gloves are changed and the implant is placed. The pocket is closed using horizontal mattress or figure-of-eight sutures from the muscle to the ADM (Figure 6). The skin edges are trimmed and the skin is closed in layers. The skin is then sealed with Dermabond and covered with Tegaderm. Biopatches and tegaderm are placed around the drains. Microfoam tape is placed to stabilize implant position. The tape is covered with Tegaderm to allow postoperative showering (3,9).
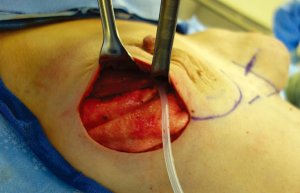
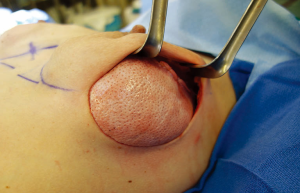
Postoperative management
The patient stays in the hospital one or two nights. No bra is placed for the first 12 hours. Prior to discharge from the hospital a loose-fitting surgical bra is placed to help support the implants. A tight compressive bra or wrap is avoided as it may compromise blood flow to the breast skin. Drains are removed when output is less than 30 cc for 24 hours. Typically one drain is removed from each side one week after surgery and the other two drains are removed two weeks after surgery. Patients are maintained on oral antibiotics until the drains are removed. If Tegaderm is covering the incisions, patients may shower and let water run over the Tegaderm dressing. Activity is limited for 4-6 weeks after surgery. A patient may not lift more than 10 pounds during this time. At 4 weeks, the patient is instructed to start implant massage. This is particularly important for smooth round implants to help avoid contracture.
Complications and management
Skin necrosis: ischemic injury to the breast skin may occur during the mastectomy if the skin flaps are made too thin or if there is excessive traction on the skin flaps. This is often seen as exposed dermis on the undersurface of the flap and/or a red/blue discoloration to the skin immediately after the mastectomy, or with inflation of a sizer. If significant ischemic injury is observed at the time of surgery, it is best to do a 2-stage or delayed breast reconstruction. Ischemic injury may also occur during reconstruction as placement of an implant can put direct stress on the skin flaps limiting perfusion. Ischemic injury may result in skin necrosis. If skin necrosis ensues, it is best managed aggressively. Skin edge necrosis (2-5 mm) can often be managed with debridement and closure under local anesthesia. If the necrosis is more severe, the implant may need to be downsized or changed to a tissue expander.
Infection: following mastectomy, the skin inevitably experiences some element of reduced blood supply making it more susceptible to infections. An infection presents as redness of the skin (cellulitis), fever, increased pain, swelling, or a combination of the above. The initial step in management is antibiotics (10). For patients presenting with redness of the skin without other symptoms or comorbidities, oral antibiotics are administered. If other symptoms are present, or if the redness does not improve with oral antibiotics, the patient is placed on intravenous antibiotics. Failure to respond to intravenous antibiotics results in an operation for implant exchange or removal (explant). If the inside pocket appears clean without evidence of infection, consideration can be made to pocket irrigation and implant replacement. If there is evidence of periprosthetic infection inside the pocket, the implant is removed and the incision is closed over a drain.
Seroma: fluid may accumulate inside the breast pocket if it is not adequately drained or if the drains are removed before the body can reabsorb the lymphatic fluid. Seromas are managed with percutaneous or operative drainage.
Other complications: potential complications include bleeding, hypertrophic scar, capsular contracture, asymmetry, implant rupture, and contour deformity.
Outcomes
A single institution study examined outcomes of 1-stage prosthetic breast reconstruction with ADM compared to 2-stage reconstruction without ADM (3). In this series of 331 DTI reconstructions, there was no difference in overall or individual complications compared to 2-stage reconstruction. There was a learning curve in complication rates with fewer complications observed as the surgeons gained experience with the technique. Patients with preoperative or postoperative radiation had an increase in complications. Patient satisfaction was assessed retrospectively with the Breast-Q. Survey results showed a similar high degree of satisfaction in 1-stage compared to 2-stage reconstruction (unpublished data).
In another large series of 260 patients and 466 breasts, the complication rate was low at 3.9% and the explant rate was 1.3% (11). The authors conclude DTI reconstruction with ADM is safe and reliable.
Conclusions
1-stage prosthetic breast reconstruction is a safe, reliable way to reconstruct the breast. With proper patient selection and surgical judgment, an aesthetically pleasing breast can be created in one surgery combined with the mastectomy and achieve results similar to traditional 2-stage surgery.
Acknowledgments
Disclosure: Amy S. Colwell, MD, is a consultant for Lifecell and Allergan.
References
- Available online: http://www.cancer.org/cancer/breastcancer/index
- Fitzpatrick AM, Gao LL, Smith BL, et al. Cost and outcome analysis of breast reconstruction paradigm shift. Ann Plast Surg 2014;73:141-9. [PubMed]
- Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg 2011;128:1170-8. [PubMed]
- Available online: www.plasticsurgery.org
- Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg 2014;133:496-506. [PubMed]
- Lin A, Liao E, Winograd J, et al. Nipple-Sparing mastectomy in patients with previous breast surgery: comparative analysis of 123 immediate reconstructions. PSRC 59th Annual Meeting 2014;Abstract 98.
- Reish RG, Lin A, Austen WG, et al. Breast reconstruction outcomes after nipple-sparing mastectomy and radiation therapy. PSRC 59th Annual Meeting 2014;P32.
- Colwell AS, Gadd M, Smith BL, et al. An inferolateral approach to nipple-sparing mastectomy: optimizing mastectomy and reconstruction. Ann Plast Surg 2010;65:140-3. [PubMed]
- Scheflan M, Colwell AS. Tissue Reinforcement in Implant-based Breast Reconstruction. Plast Reconstr Surg Glob Open 2014;2:e192. [PubMed]
- Reish RG, Damjanovic B, Austen WG Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg 2013;131:1223-30. [PubMed]
- Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg 2011;127:514-24. [PubMed]


