
Analysis of primary and secondary squamous cell carcinoma of the thyroid gland: a retrospective study
Introduction
Squamous cell carcinoma of the thyroid (SCCT) is a rare malignant disease and includes primary squamous cell carcinoma of the thyroid (PSCCT) and secondary squamous cell carcinoma of the thyroid (SSCCT) based on the origination. PSCCT is a rare carcinoma, constituting less than 1% of all thyroid malignancies (1). It was first reported by von Karst in 1958 (1,2). Although half a century has passed, the etiology of PSCCT is still unknown. Some researchers have indicated that the squamous cells originated from metaplasia complicated with chronic thyroid diseases (3). Some other studies reported that the squamous epithelium originated from the ultimobranchial bodies or the thyroglossal duct (4). The PSCCT patients are always diagnosed in the fifth or sixth decades of life (5-7). Most related studies suggest that PSCCT is an aggressive cancer, and most patients die of airway compromise (1,6,7). Age, tumor grade, and tumor size are related to overall survival (OS) and disease-specific survival (DSS) (5). Early diagnosis, radical operation, and adjuvant radiation therapy seem to be associated with a better outcome (6). SSCCT is a type of squamous cell carcinoma that metastasizes to the thyroid. Although there are only a few reports of SSCCT, it is postulated that adjacent organs, including the esophagus, trachea, and larynx, are the most common primary sites (8,9). Some studies have demonstrated that SSCCT is more commonly seen and has a better outcome than PSCCT (7,8). One of the principles for distinguishing SSCCT from PSCCT is to find the primary tumor. Thyroid transcription factor (TTF) and thyroglobulin of the pathology could also help to differentiate those two diseases (7). Because of their scarcity, there have only been a few reports of SCCT in the world, and most of them were reviews of several databases. Until now, there have been few studies with more details of the clinical manifestations. It is very valuable to predict the prognosis based on the clinical manifestations. In this study, we reviewed the clinical characteristics of SCCT patients in China. We compared the clinical symptoms and ultrasound and pathological characteristics, aiming to gain a better understanding of the diagnosis and prognosis of SCCT. We present the following article in accordance with the STROBE reporting checklist (available at
Methods
Patients and materials
Patients diagnosed with PSCCT and patients diagnosed with SSCCT were enrolled at the Peking Union Medical College Hospital (PUMCH) from March 1992 to October 2019. The inclusion and exclusion criteria of PSCCT were as follows (5,10).
Inclusion criteria: (I) patients diagnosed as PSCCT with pathological evidence from biopsy or resection specimen; (II) patients with complete medical records. Exclusion criteria: (I) patients with known primary cancer of other sites; (II) patients with incomplete medical records.
All the SSCCT patients were diagnosed as thyroid metastases from other primary sites. Clinical information was collected from the medical records, including gender, age, carcinoma origination, chief complaint, weight loss, complicated chronic disease, cigarette use, related physical examination, ultrasound, and laryngoscope manifestation. Information including treatment, pathological signs and outcome was also collected. Well-differentiated, moderately differentiated and poorly differentiated classification of Squamous cell carcinoma (SCC) was used to estimate the differentiation grade of SCCT. All of the patients were reviewed by at least two experienced surgeons. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the Peking Union Medical College Hospital (No. JS-2555) and individual consent for this retrospective analysis was waived.
Statistical analysis
Data were analysed using SPSS software (version 16.0, SPSS Inc., Chicago, IL). According to their characteristics, the differences were evaluated using the Fisher test, the χ2 test, or the independent samples t-test. An OS curve was constructed using the Kaplan-Meier method. Univariate survival analysis was calculated via the log-rank test. Factors with log-rank P values less than 0.15 in the univariate analysis were selected for multivariate survival analysis using a Cox regression model. Patients with missing data were excluded in each subgroup analysis. P values less than 0.05 were considered statistically significant.
Results
Basic clinical characteristics
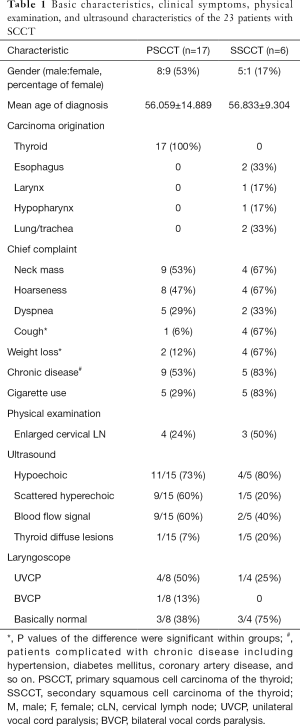
There were 17 patients diagnosed with PSCCT (8 males and 9 females; median age 56.059±14.889 years; ranging from 31 to 83 years) and 6 patients diagnosed with SSCCT (5 males and 1 female; median age 56.833±9.304 years; ranging from 45 to 67 years) (Table 1). There were no significant differences in gender and age between the two groups. The primary sites of SSCCT were the esophagus (33%), larynx (17%), hypopharynx (17%) and lung/trachea (33%).
Full table
Symptoms and imaging characteristics
The most common chief complaint was neck mass in both groups (53% in PSCCT group and 67% in SSCCT group) (Table 1). Approximately 47% of the PSCCT patients also complained of hoarseness, and 67% of the SSCCT patients complained of hoarseness and cough. The percentage of patients with cough was higher in the SSCCT group than in the PSCCT group (P=0.008). Approximately 29% of the PSCCT patients and 33% of the SSCCT patients complained of dyspnea when they came to the hospital. Weight loss was a complaint of 12% of patients in the PSCCT group and 67% of patients in the SSCCT group (P=0.021). Approximately 53% of PSCCT patients and 83% of SSCCT patients were complicated with chronic diseases, such as hypertension, diabetes mellitus, coronary artery disease. A total of 29% of PSCCT patients and 83% of SSCCT patients used cigarettes. Approximately 24% of PSCCT patients and 50% of SSCCT patients had enlarged cervical lymph nodes during the physical examination in the hospital.
Two of the PSCCT patients and one of the SSCCT patients had no complete ultrasound examination results. According to the ultrasound, most of the lesions were hypoechoic in both groups. Approximately 60% of lesions in the PSCCT patients were scattered hyperechoic and had blood flow signals, while 20% of lesions in the SSCCT patients were scattered hyperechoic, and 40% of lesions had blood flow signals. Thyroid diffuse lesions could also be seen in 7% PSCCT patients and 20% SSCCT patients. Eight of the PSCCT patients and four of the SSCCT patients underwent laryngoscope examination. Based on the laryngoscopy results, vocal cord paralysis was more commonly seen in PSCCT patients (Table 1).
Treatment and pathological features
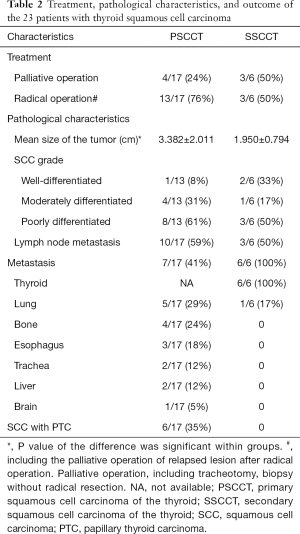
In the PSCCT patients, 24% underwent a palliative operation, and 76% underwent a radical operation (Table 2). In the patients with a radical operation, three relapsed and could only undergo a palliative operation. In the SSCCT patients, 50% underwent a radical operation, and 50% underwent a palliative operation.
Full table
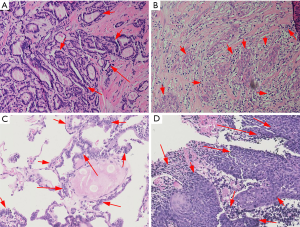
According to the pathological characteristics, we found that the mean size of the tumor in the PSCCT group was 3.382±2.011 cm, and the mean size of the tumor in the SSCCT group was 1.950±0.794 cm. The difference in size was significant (P=0.024). We also compared the grade of each carcinoma, four PSCCT patient were excluded because of difficulty with classification. We found that there was a lower percentage of well-differentiated grade in the pathology of the PSCCT group (8%) than in the SSCCT group (33%). More than half of the patients had lymph node metastasis combed with clinical manifestations. Although we excluded other primary sites of the PSCCT patients at diagnosis, we also found that the PSCCT could metastasize to the lung (29%), bone (24%), esophagus (18%), trachea (12%), liver (12%) and brain (5%) in the follow-up. Furthermore, we found that six PSCCT patients (36%) had papillary thyroid carcinoma (PTC) (Figure 1). Moreover, two of them had two carcinomas at the same time, and four of them found SCCT after recurrent PTC, which may support the hypothesis that the squamous cells originated from a metaplasia complicated with chronic thyroid diseases.
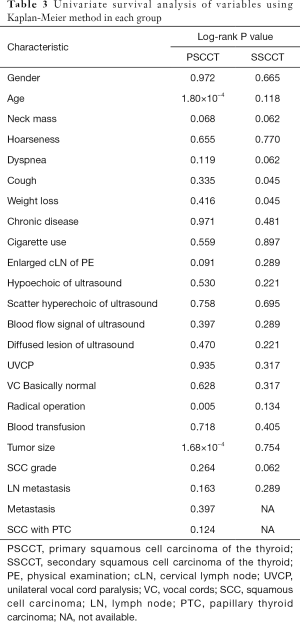
According to Kaplan-Meier survival analysis, the mean survival times after diagnosis were 17.053±3.361 months for the PSCCT patients and 13.500±5.216 months for the SSCCT patients (Figure 2). There was no significant difference between these two groups (P=0.843). The mean survival times were 17.053 months (95% CI: 10.466–23.641) in the PSCCT patients and 13.500 months (95% CI: 3.276–23.724) in the SSCCT patients. The median survival times were 12 months (95% CI: 8.137–15.863) in the PSCCT patients and 3 months (95% CI: 0.00–12.602) in the SSCCT patients. We also conducted univariate survival analysis of variables using the Kaplan-Meier method in each group. The survival time was significantly different based on age (log-rank P=1.80×10−4), radical operation (log-rank P=0.005), and tumor size (log-rank P=1.68×10−4) in PSCCT (Table 3). The survival time manifested with a statistically significant difference based on cough (log-rank P=0.045), and weight loss (log-rank P=0.045) in SSCCT.
Full table
In the multivariate analysis, we assessed the factors with log-rank p values less than 0.15 in the univariate analysis. Using a Cox regression model, we found that the following factors were independent predictors of PSCCT OS: age at diagnosis (HR 1.251, 95% CI: 1.036–1.510, P=0.020), enlarged cLN of PE (HR 47.560, 95% CI: 1.995–1133.772, P=0.017), radical operation (HR 1.06×10−3, 95% CI: 9×10−6–0.124, P=4.82×10−3), and PTC (HR 34.448, 95% CI: 1.402–846.333, P=0.030) (Table 4). There were no significant independent predictors of OS in the SSCCT patients by multivariate survival analysis.

Full table
Discussion
SCCT is an aggressive malignant disease that includes PSCCT and SSCCT. Bothe types have an extremely poor prognosis (11,12). PSCCT is rarely seen in the clinic, and only hundreds of cases have been reported in the literature, most of which were from the review of databases. Along with the increasing frequency of thyroid regular examination, SSCCT could be a type of metastatic malignancy mimicking PSCCT.
In this study, we reviewed the clinical data of PSCCT and SSCCT patients in China. Most of the patients were approximately 50 to 60 years old in both groups. According to previous studies, most PSCCT patients were more than 60 years old (5,6,11). The percentage of female PSCCT patients was slightly higher than that of males PSCCT patients in our study, which is consistent with previous studies (5,6,11). Since the sample size of our study was limited, further related studies with larger sizes are needed to identify the tendency of gender, especially in SSCCT. We also identified the primary sites of involvement in SSCCT patients. Most of the primary sites were from the adjacent organs from the neck. From the literature review, carcinoma from other organs could also metastasize to the thyroid, including the colon, skin, kidney, uterus, stomach, bone marrow, and prostate (8). However, the squamous cell carcinoma cell type could only be seen in the lung and other organs from the head and neck (8,9).
We also found that neck mass was the most common chief complaint among PSCCT and SSCCT patients in our study, which might be caused by the rapid enlargement of the carcinoma. Hoarseness were also commonly seen in both groups. We hypothesized that the recurrent laryngeal nerve was easily influenced by the malignancy because of its immediate vicinity and the tumor’s invasiveness. Cough and weight loss were more commonly seen in SSCCT patients, which we hypothesized were determined by the primary site of the carcinoma. The symptoms and physical examinations of SCCT patients revealed distinguishing features, and ultrasound could provide some help in diagnosis. A hypoechoic sign on ultrasound was the most common feature of the lesions. Some of the PSCCT lesions also manifested with scattered hyperechoic signal and blood flow signal. Previous studies also indicated that eggshell calcification could be seen in the sonographic findings of PSCCT (13).
Our study results also showed that the pathological characteristics and outcomes were poorer in the PSCCT patients. Several PSCCT patients relapsed and could only undergo a palliative operation, even after a radical operation. The tumor size was larger, the SCC grade was lower, and more patients with PSCCT had lymph node metastases. In accordance with literature studies, we confirmed that the PSCCT could metastasize to other organs due to the invasiveness of PSCCT (6,14).
Consistent with previous reports, our study also confirmed that PTC could recur as SCCT (12,15). Approximately 35% of PSCCT patients had PTC, and two-thirds of them were diagnosed with PSCCT after recurrent PTC. Thus, regular examination is necessary for patients with PTC, especially for those with recurrence.
We found that the prognosis of SCCT patients was poor, with a short median survival time, although SSCCT is a type of metastatic disease, and its malignancy mainly depends on the primary site. Previous studies also found that PSCCT is more aggressive than SSCCT (7). Our study indicated that the mean and median survival times were longer in PSCCT patients than in SSCCT patients. We also found that age at diagnosis, enlarged cervical lymph nodes in the physical examination, and PTC were independent risk predictors of OS in PSCCT. Radical operation was a protective independent predictor of OS in PSCCT. Further research is needed to explore the underlying mechanism. As this study was limited to the sample size of SSCCT patients, no independent predictors were found in SSCCT using multivariate survival analysis.
There are several limitations in our study. First, according to the literature, additional tests could improve the diagnosis rate, such as BRAF mutation testing (16), PET/CT (17), and immunoreactions including p21, MIB-I, p53, thyroglobulin and TTF (7,18). Although we excluded other primary sites of PSCCT using CT, laryngoscope, and upper gastrointestinal contrast at diagnosis, more techniques should be added to improve the accuracy. Second, the number of patient sample was small. The significance of this analysis could be influenced as a result, especially for the SSCCT group. Thus, larger samples are needed for replication.
Conclusions
In conclusion, we described the largest cohort of PSCCT in one single center in China. SCCT is aggressive, with neck mass being the most common chief complaint. There were several different characteristics between PSCCT and SSCCT. A hypoechoic signal with a scattered hyperechoic sign of ultrasound supports the diagnosis. PTC could be combined with or recurrent as PSCCT. Age, enlarged cervical lymph nodes, radical operation, and PTC were the predictors of OS in PSCCT patients. These characteristics can help elucidate the diagnosis and prognosis of SCCT.
Acknowledgments
We give our great thanks to the patients for their active participation.
Funding: This research was funded in part by the National Natural Science Foundation of China (81902271 to G Liu).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-628
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-628
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-628). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of the Peking Union Medical College Hospital (No. JS-2555) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang TY, Assor D. Primary squamous cell carcinoma of the thyroid gland: a report of four cases. Am J Clin Pathol 1971;55:93-8. [Crossref] [PubMed]
- Zhou XH. Primary squamous cell carcinoma of the thyroid. Eur J Surg Oncol 2002;28:42-5. [Crossref] [PubMed]
- Goldman RL. Primary squamous cell carcinoma of the thyroid gland: report of a case and review of the literature. Am Surg 1964;30:247-52. [PubMed]
- Kampsen EB, Jager N, Max MH. Squamous cell carcinoma of the thyroid: a report of two cases. J Surg Oncol 1977;9:567-78. [Crossref] [PubMed]
- Au JK, Alonso J, Kuan EC, et al. Primary Squamous Cell Carcinoma of the Thyroid: A Population-Based Analysis. Otolaryngol Head Neck Surg 2017;157:25-9. [Crossref] [PubMed]
- Yang S, Li C, Shi X, et al. Primary Squamous Cell Carcinoma in the Thyroid Gland: A Population-Based Analysis Using the SEER Database. World J Surg 2019;43:1249-55. [Crossref] [PubMed]
- Booya F, Sebo TJ, Kasperbauer JL, et al. Primary squamous cell carcinoma of the thyroid: report of ten cases. Thyroid 2006;16:89-93. [Crossref] [PubMed]
- Michelow PM, Leiman G. Metastases to the thyroid gland: diagnosis by aspiration cytology. Diagn Cytopathol 1995;13:209-13. [Crossref] [PubMed]
- Vatsyayan A, Mandlik D, Patel P, et al. Metastasis of squamous cell carcinoma of the head and neck to the thyroid: a single institution's experience with a review of relevant publications. Br J Oral Maxillofac Surg 2019;57:609-15. [Crossref] [PubMed]
- Lam AK. Squamous cell carcinoma of thyroid: a unique type of cancer in World Health Organization Classification. Endocr Relat Cancer 2020;27:R177-92. [Crossref] [PubMed]
- Limberg J, Ullmann TM, Stefanova D, et al. Prognostic Characteristics of Primary Squamous Cell Carcinoma of the Thyroid: A National Cancer Database Analysis. World J Surg 2020;44:348-55. [Crossref] [PubMed]
- Dennis K, O'Neil M, Harrington A. Not all neck mass fine-needle aspirations with squamous cells are squamous cell carcinoma; report of a case of recurrent thyroid carcinoma with papillary and squamous components. Cytojournal 2018;15:23. [Crossref] [PubMed]
- Chen CY, Tseng HS, Lee CH, et al. Primary squamous cell carcinoma of the thyroid gland with eggshell calcification: sonographic and computed tomographic findings. J Ultrasound Med 2010;29:1667-70. [Crossref] [PubMed]
- Harada T, Shimaoka K, Yakumaru K, et al. Squamous cell carcinoma of the thyroid gland -- transition from adenocarcinoma. J Surg Oncol 1982;19:36-43. [Crossref] [PubMed]
- Kitahara S, Ito T, Hamatani S, et al. Thyroid papillary carcinoma recurring as squamous cell carcinoma: report of a case. Surg Today 2006;36:171-4. [Crossref] [PubMed]
- Basnet A, Pandita A, Fullmer J, et al. Squamous Cell Carcinoma of the Thyroid as a Result of Anaplastic Transformation from BRAF-Positive Papillary Thyroid Cancer. Case Rep Oncol Med 2017;2017:4276435. [Crossref] [PubMed]
- Cai L, Chen Y, Huang Z, et al. Primary squamous cell carcinoma of the thyroid on FDG PET/CT. Clin Nucl Med 2014;39:1014-6. [Crossref] [PubMed]
- Fassan M, Pennelli G, Pelizzo MR, et al. Primary squamous cell carcinoma of the thyroid: immunohistochemical profile and literature review. Tumori 2007;93:518-21. [Crossref] [PubMed]



