
Application of ultrasonic shear wave elastography and contrast-enhanced ultrasound in the differential diagnosis of patients with benign and malignant thyroid lesions
Introduction
Thyroid nodules are a common thyroid disorder, which can be manifested in thyroid inflammation, tumors, and autoimmune diseases. It has been reported that 4–8% of adults have palpable thyroid nodules, most of which are benign, while about 5% are malignant (1). Studies have also shown that the clinical incidence of thyroid cancer is increasing annually, and in areas exposed to iodine radiation, the probability of patients with malignant thyroid nodules is about 20–30% (2). Thyroid cancer in usually asymptomatic in the early stage. Therefore, it is important to differentiate between benign and malignant nodules quickly and accurately and to develop suitable treatment modalities to prevent delays in the diagnosis of thyroid cancer. Needle aspiration biopsy is the gold standard for the diagnosis of benign and malignant thyroid nodules; however, it is an invasive examination technique, and 10–15% of nodules cannot be clearly diagnosed by this method (3). Imaging examinations are the main diagnostic techniques for thyroid nodules. Among them, computed tomography (CT) and Magnetic Resonance Imaging (MRI) are both non-invasive methods. Although they have certain advantages in the evaluation of the position, shape, and calcification of nodules, but are not routinely used due to various reasons, including high cost. Ultrasound is also a common non-invasive technique in clinical practice with obvious advantages, such as low cost, easy operation, and high accuracy, and is considered the preferred imaging examination technique for thyroid nodules (4). However, the conventional ultrasound cannot clearly show tracheal infiltration, nodules more than 4 cm and retrosternal lesions. Ultrasonic shear wave elastography (SWE) and contrast-enhanced ultrasound (CEUS) can dynamically and quantitatively observe corresponding tissue enhancement mode and hardness, which can effectively make up for the lack of conventional ultrasound. However, there are few comparative studies on the identification efficiency of thyroid nodules between SWE and CEUS. Therefore, the aim of the present study was to explore the application value of SWE and CEUS in the differential diagnosis of benign and malignant thyroid nodules. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/gs-20-819).
Methods
Clinical data
A total of 82 patients with thyroid nodules (96 thyroid nodules) admitted to the Affiliated Hospital of North Sichuan Medical College from February 2018 to June 2019 were selected as study participants. Thyroid nodules were confirmed by surgery and pathological diagnosis. The inclusion criteria were as follows: (I) thyroid nodules found in the preoperative examination, with the smallest diameter ≥0.5 cm; (II) patients with corresponding surgical indications of thyroid nodules and who accepted the surgical treatment; (III) SWE and CEUS examinations performed prior to surgery, with complete examination data; and (IV) patients provided signed informed consent to participate in the present study. The exclusion criteria were as follows: (I) pregnant or lactating patients; (II) patients with a history of allergy to sulphur hexafluoride or other components of SonoVue; (III) patients with hematological diseases or autoimmune dysfunction; and (IV) patients with mental illness who were unable to cooperate with treatment. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Affiliated Hospital of North Sichuan Medical College (NO.: 2019ER(A)024). And informed consent was taken from all the patients.
SWE inspection
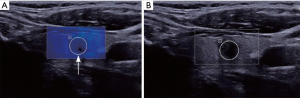
The inspection was carried out using an SWE ultrasonic diagnostic apparatus (AixPlorer; SuperSonic Imagine, Aix-en-Provence, France), with the L4–15 linear array probe at a frequency of 4–15 MHz. The patient was placed in the the supine position, and 2D grayscale ultrasound and color Doppler ultrasound examination was performed with preset thyroid scan parameters. After routine scanning, nodule position, size, appearance, boundary conditions, echo of the nodule, and blood flow conditions were observed and recorded. SWE was then initiated. The probe was routinely moved without pressurization. After locating the target nodule, the patient was instructed to hold their breath for about 3–5 s until the image was stable. The image was then taken, and the Young’s modulus value of the nodules was measured in the region of interest through the Q-BOX function. The region of interest was generally selected in the default sampling frame in the ultrasonic instruments and covered the harder part of the lesion as much as possible. For nodules with liquid, the solid part of the nodules should be taken. Using this technology, the absolute value of the Young’s modulus was obtained, and the elastic map could be effectively transformed into a visualized acoustic image through computer processing. According to the standards, blue indicated that the hardness of the tissue was low, and red indicated that the hardness of the tissue was high. The result of the average Young’s modulus value for each nodule was automatically calculated. For each lesion, Young’s modulus value for each nodule needed to be measured 3 times to calculate the average value based on the results.
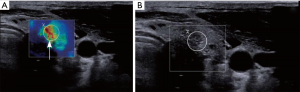
For the CEUS examination, the Acuson Sequoia 512 ultrasound system (SIEMENS AG FWB, Berlin, Germany) was selected, with a 15L8W broadband linear array probe. First, a 2D ultrasound examination was performed to determine the nodule position, size, echo, boundary, calcification for the color Doppler flow imaging. CEUS examination was performed by comparing the pulse sequence, with a probe transmission frequency of 7.0 MHz and a mechanical index of 0.32. SonoVue contrast agent (2.4 mL; Bracco SpA, Milan, Italy) was injected via superficial vein bolus, and then 5 mL saline was injected to flush the piping. The patient was instructed to keep breathing normally and to not swallow. After completing the contrast injection operation, the timer and the dynamic storage buttons were started, and the acquired image was observed and stored on hard disk. Each image was analyzed by 2 experienced sonographers. If there were inconsistences, they discussed together until consensus was reached. Images with circular enhancement were considered to be benign, and images with inhomogeneous enhancement were considered to be malignant. All patients were examined by the same experienced sonographer.
Observation indicators
The average Young’s modulus value, CEUS imaging characteristics, and quantitative parameters were compared between the benign and malignant groups using surgical pathology as the gold standard. The receiver-operating characteristic (ROC) curve was used to analyze the diagnostic efficiency of SWE, CEUS, and a combination of both.
Statistical analysis
The index database was constructed in Excel (IBM, USA), and the data were analyzed using SPSS software (IBM, New York, USA). Normal distribution measurement data were expressed as
Results
Comparison of general data between the benign and malignant groups
Surgical pathology found that nodules of 52 cases were benign (n=62 nodules) and 30 were malignant (n=34 nodules); these comprised the benign group and the malignant group, respectively. There was no significant difference in general data, such as sex, age, body mass index, and maximum diameter of nodules between the 2 groups (P>0.05) (Table 1).
Comparison of the average Young’s modulus value between the benign and malignant groups
The average Young’s modulus value in the malignant group nodule was significantly higher than that of the benign group nodule (P<0.05) (Table 2). The SWE images of typical thyroid nodules are shown in Figures 1,2.

Full table
Comparison of CEUS images between the benign and malignant groups
There were significant differences in the border, morphology, perfusion intensity, homogeneous enhancement, and perfusion defect status in CEUS images between the benign and malignant groups (P<0.05) (Table 3). The CEUS images of typical thyroid nodules are shown in Figures 3,4.

Full table
Comparison of quantitative parameters of angiographic examination between the benign and malignant groups
There was no significant difference in the initial increase time, peak intensity, peak time, and area under the curve between the 2 groups (P>0.05). The curve sharpness in the benign group was significantly lower than that of the malignant group (P<0.05) (Table 4).
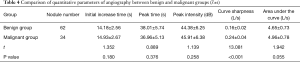
Full table
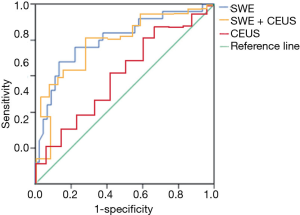
Diagnostic value of SWE and CEUS
The area under the curve of SWE, CEUS, and their combination is shown in Table 5. The diagnostic sensitivity and specificity of SWE, CEUS, and their combination was 90.1% and 81.6%, 67.8% and 75.4%, and 97.3% and 71.5%, respectively (Figure 5).
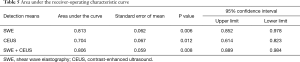
Full table
Discussion
CEUS can provide richer lesion vascular distribution and blood flow for disease diagnosis by enhancing tumor blood vessels and increasing the contrast of tumor blood vessels after injecting contrast agents (5,6). Compared with benign tumors, the blood supply of malignant tumors is more abundant, and they generally have significant differences in the number, structure, and morphology of blood vessels. Therefore, CEUS has positive significance for the differential diagnosis of benign and malignant thyroid diseases (7). According to the findings of the present study, benign thyroid nodules mostly manifested as lesions with clear borders and regular morphology, and most of the nodules had circular enhancements around them. This phenomenon may be closely related to the corresponding ring blood flow signal, which is enhanced by ultrasound contrast agent. Benign thyroid nodules mainly have rapid filling, usually characterized as “fast in and slow out”. CEUS imaging showed homogeneous enhancement and no perfusion defects. However, the initial increase time of malignant nodules was delayed compared with adjacent tissues, and the clearance time was earlier than adjacent tissues with inhomogeneous enhancement performance. The enhancement degree of malignant nodules was lower than that of adjacent tissues, indicating “slow in and fast out”. In a CEUS quantitative analysis, the time-intensity curve was used to effectively reflect the change in characteristics of the contrast agent in the lesion over time during the imaging process, and to obtain quantitative parameters (8,9). In the present study, there was no significant difference between benign and malignant thyroid nodules in the initial increase time, peak time, area under the curve, and peak intensity; however, the curve sharpness of malignant nodules was significantly higher than that of benign nodules. In a previously published quantitative analysis of the time-intensity curve during CEUS examination, the sensitivity and specificity for diagnosing benign and malignant thyroid diseases were 76.9% and 84.8%, respectively (10). In the present study, based on the inhomogeneous enhancement standard, the ROC curve analysis showed that the diagnostic sensitivity and specificity of CEUS were 67.8% and 75.4%, respectively, indicating that CEUS has a certain diagnostic value for benign and malignant thyroid diseases. At present, thyroid diagnosis with CEUS is still in the preliminary exploratory stage. The inconsistent results of relevant research may be related to inconsistencies in evaluation indicators (i.e., peak intensity of the fitted curve, signs, average passage time of contrast agent, and average perfusion intensity). Therefore, the value of CEUS in the diagnosis of benign and malignant diseases remains to be verified by further studies with larger sample sizes.
Ultrasound elastography is a new diagnostic technology that has been developed in recent years, which can be used to distinguish benign and malignant thyroid diseases. Elastography generally adopts the area score method to determine the ratio of strain rate between the tumor and adjacent tissues, which is a semiquantitative examination of nodule hardness. SWE is a type of quantitative measurement technology, and the Young’s modulus value measured by it is the elastic modulus of the object; that is, the ratio of stress to strain. The stress and strain are proportional within the elastic limit of the object, and the ratio can reflect the elasticity of the organization; that is, the greater the Young’s modulus value, the harder the structure (11,12). Previous pathological studies have found that the elastic coefficient of tissues, such as fat, breast tissue, fibrotic tissue, and non-invasive cancerous tissue, are significantly different from invasive cancerous tissue (13). Human benign thyroid lesions, such as adenomas and nodular goiters, are mainly composed of follicular cells, which are soft due to the glial components in the cell body. In contrast, the hardness of malignant thyroid lesions, such as papillary thyroid carcinoma, is high due to multiple branches of the tissue and calcified bodies, fibers, and blood vessels in the stroma (14,15). The SWE inspection method uses modern technologies, such as acoustic radiation pulse control, multi-wave imaging, and fusion frequency conversion, which can effectively gather at different depths of the tissue, and finally generate shear waves. Because the elasticity of the tissue and the propagation rate of the shear waves are positively correlated, SWE could be used to quantitatively detect the Young’s modulus value. In the present study, the average Young’s modulus value of malignant nodules was significantly higher than that of benign nodules, which was consistent with Lam et al.’s findings (16), indicating that there is a difference in the average Young’s modulus value between benign and malignant thyroid nodules, and this can be used to distinguish benign and malignant nodules. It has been reported that SWE has a sensitivity of 90% and a specificity of 80% in the diagnosis of benign and malignant thyroid nodules (17). In the present study, the diagnostic sensitivity and specificity of SWE were 90.1% and 81.6%, respectively, which were consistent with the earlier reported results. Compared to CEUS, SWE showed higher diagnostic sensitivity and specificity. In the present study, sensitivity and specificity of the combined diagnosis of CEUS and SWE were 97.3% and 71.5%, respectively, indicating that CEUS combined with SWE can improve the sensitivity of SWE alone, but with poor specificity. It is recommended that clinical indicators such as serum thyrotrophin and neutrophil/lymphocyte ratio (NLR) be combined to improve diagnostic specificity. However, the application of CEUS and SWE also has certain limitations, such as high requirements on ultrasonic machines, so it is difficult to promote and apply them in grassroots hospitals at present.
In summary, compared with CEUS, SWE is more effective in diagnosing benign and malignant thyroid nodules. A combination of both can increase the sensitivity of SWE diagnosis and can effectively increase diagnostic efficiency. However, the limitations of the present study included the small sample size and that it was a non-multicenter study. Therefore, future multicenter studies with larger sample sizes are warranted to confirm the findings of the present study.
Acknowledgments
Funding: This work was supported by the Scientific Research Project of Sichuan Health Committee (grant no. 19PJ036).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-819
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-819
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-819). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by The Affiliated Hospital of North Sichuan Medical College (NO.: 2019ER(A)024). And informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoang JK, Middleton WD, Farjat AE, et al. Reduction in Thyroid Nodule Biopsies and Improved Accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology 2018;287:185-93. [Crossref] [PubMed]
- Kitahara CM, Preston DL, Neta G, et al. Occupational radiation exposure and thyroid cancer incidence in a cohort of U.S. radiologic technologists, 1983-2013. Int J Cancer 2018;143:2145-9. [Crossref] [PubMed]
- Suh CH, Baek JH, Lee JH, et al. The role of core-needle biopsy in the diagnosis of thyroid malignancy in 4580 patients with 4746 thyroid nodules: a systematic review and meta-analysis. Endocrine 2016;54:315-28. [Crossref] [PubMed]
- Cui K, Zhang M, Fu C, et al. Comment on “Partially Cystic Thyroid Nodules on Ultrasound: Probability of Malignancy and Sonographic Differentiation”. Thyroid 2016;26:1645. [Crossref] [PubMed]
- Bhatia P, Deniwar A, Friedlander P, et al. Diagnostic potential of ancillary molecular testing in differentiation of benign and malignant thyroid nodules. Anticancer Res 2015;35:1237-41. [PubMed]
- Zhou X, Zhou P, Hu Z, et al. Diagnostic Efficiency of Quantitative Contrast-Enhanced Ultrasound Indicators for Discriminating Benign From Malignant Solid Thyroid Nodules. J Ultrasound Med 2018;37:425-37. [Crossref] [PubMed]
- Ma X, Zhang B, Ling W, et al. Contrast-enhanced sonography for the identification of benign and malignant thyroid nodules: Systematic review and meta-analysis. J Clin Ultrasound 2016;44:199-209. [Crossref] [PubMed]
- Zhang YZ, Xu T, Gong HY, et al. Application of high-resolution ultrasound, real-time elastography, and contrast-enhanced ultrasound in differentiating solid thyroid nodules. Medicine (Baltimore) 2016;95:e5329. [Crossref] [PubMed]
- Wu Q, Wang Y, Li Y, et al. Diagnostic value of contrast-enhanced ultrasound in solid thyroid nodules with and without enhancement. Endocrine 2016;53:480-8. [Crossref] [PubMed]
- Liu Q, Cheng J, Li J, et al. The diagnostic accuracy of contrast-enhanced ultrasound for the differentiation of benign and malignant thyroid nodules: A PRISMA compliant meta-analysis. Medicine (Baltimore) 2018;97:e13325. [Crossref] [PubMed]
- Ng WL, Rahmat K, Fadzli F, et al. Shearwave Elastography Increases Diagnostic Accuracy in Characterization of Breast Lesions. Medicine (Baltimore) 2016;95:e3146. [Crossref] [PubMed]
- Thiele M, Detlefsen S, Sevelsted Møller L, et al. Transient and 2-Dimensional Shear-Wave Elastography Provide Comparable Assessment of Alcoholic Liver Fibrosis and Cirrhosis. Gastroenterology 2016;150:123-33. [Crossref] [PubMed]
- Mondal U, Henkes N, Patel S, et al. Endoscopic Ultrasound Elastography: Current Clinical Use in Pancreas. Pancreas 2016;45:929-33. [Crossref] [PubMed]
- Nishino M. Molecular cytopathology for thyroid nodules: A review of methodology and test performance. Cancer Cytopathol 2016;124:14-27. [Crossref] [PubMed]
- Kwak JY. Thyroid ultrasonography for personalized approach at thyroid nodules. Endocrine 2016;52:181-2. [Crossref] [PubMed]
- Lam AC, Pang SW, Ahuja AT, et al. The influence of precompression on elasticity of thyroid nodules estimated by ultrasound shear wave elastography. Eur Radiol 2016;26:2845-52. [Crossref] [PubMed]
- Liu B, Liang J, Zheng Y, et al. Two-dimensional shear wave elastography as promising diagnostic tool for predicting malignant thyroid nodules: a prospective single-centre experience. Eur Radiol 2015;25:624-34. [Crossref] [PubMed]
(English Language Editor: R. Scott)



