Calcitonin-negative neuroendocrine tumor of thyroid gland mimicking anaplastic carcinoma: an unusual entity
Introduction
Neuroendocrine tumors (NETs) originate from mostly neural crest derived cells, and types of these cells differ according to organ throughout the body. They are mostly seen in midline organs such as esophagus, stomach, pancreas, intestine and lung (1). NETs have similar features with characteristic immunohistochemical and serum markers, but the biology of the tumor may differ according to tumor type (2). Parafollicular C cells are one of the neural crest cells migrating from neural crest to thyroid gland, located in basal cell of thyroid follicle and are only 1% of all thyroid cells giving rise to NET of thyroid gland, medullary thyroid carcinoma (MTC) (3).
MTC accounts for 5-8% of all thyroid tumors. Although most (70-80%) of cases are sporadic, a familial pattern with autosomal dominant trait is present in 20-30% cases. In hereditary pattern, tumor can be with pheochromocytoma and hyperparathyroidism such as in cases with multiple endocrine neoplasia (MEN) 2A. Also a hereditary variant in combination with pheochromocytoma, multiple mucosal neuromas and marfanoid habitus is present in MEN 2B. Familial MTC without accompanying other tumors is also possible (3). Calcitonin is a 32 amino acid peptide secreted by parafollicular C cells of thyroid regulating serum calcium levels. In MTC, calcitonin is a sensitive and specific marker for tumor diagnosis and follow-up and in all cases diagnosis of MTC should be confirmed by positive immunohistochemical staining for calcitonin (3).
In this case report we presented a case of a huge thyroid tumor descending to the level of thoracal hilar region with serum and immunohistochemically calcitonin negative features and having neuroendocrine staining properties.
Case
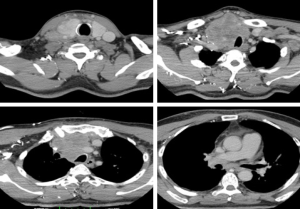
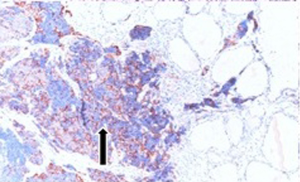
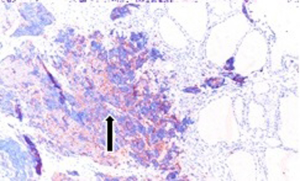
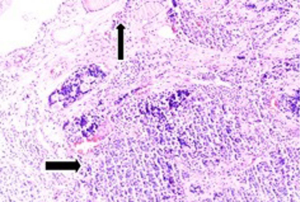
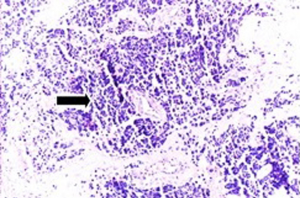
Fifty-seven years old otherwise healthy patient was consulted from general surgery department for moderate respiratory distress. In his medical history; he had a thyroid nodule for years without follow-up, there was sudden enlargement in his midline neck region for two weeks and hoarseness with moderate respiratory distress had started. He had no diarrhea or flushing complaints. In his physical examination; indirect laryngoscopic evaluation revealed paralysis of right vocal cord. Thyroid gland was diffusely enlarged, hard on palpation. A neck computerized tomography was performed. There was a huge (nearly 15 cm in sagittal section) thyroid mass starting from thyroid gland anterior to thyroid cartilage, descending intra-thoracically to the level of right hilar region with 8 cm. in width in intra-thoracic part which was thought to invade nearby anatomic structures intra-thoracically (Figure 1). Since he was in moderate respiratory distress with right vocal cord paralysis and rate of growth of the tumor was high; a tracheotomy was performed with general anesthesia. During tracheotomy; an open biopsy from thyroid tumoral tissue was performed with preliminary diagnosis of anaplastic carcinoma. The patient’s calcitonin levels was 5.6 pg/mL (>10 pg/mL for normal values) and Carcioembryogenic antigen (CEA) was also in normal limits. Histological sections of thyroid tissue (Figure 2) showed intermediate sized infiltrating malignant neoplasm composed of atypical cells with neuroendocrine chromatin (Figures 2,3). Tumor cells stained positive for chromogranin and synaptophysin (Figures 4,5) and negative for S100 protein, CEA, calcitonin, thyroglobulin and TTF-1 immunohistochemically. The tumor was diagnosed as high grade neuroendocrine carcinoma. Ki67 proliferation index was as high as 70%. RET mutation analysis couldn’t be achieved since it was available in few centers in Turkey and was very expensive than the patient could pay. Serum levels of chromogranin-A was 96 ng/mL (normal values are <94 ng/mL and normal median level is 44 ng/mL).
Follow-up of the patient
For systemic metastases 18FloroDeoxyGlucose Positron Emission Tomography (18FDG PET) was planned. There was intra-abdominal peritoneal metastatic foci. Since the tumor had invasive features in intra-thoracic region and metastases to peritoneum; it was considered as an unresectable thyroid carcinoma and chemotherapy including cysplatin and etoposide was started by Medical Oncology department. After four cure chemotherapy regimen patient is still followed-up with Medical Oncology department.
Discussion
Thyroid malignancies are mainly papillary, follicular, anaplastic, thyroid lymphoma and MTC. Diagnosis of the primary thyroid malignancy is crucial, since all these tumors have different biological behaviours and treatments (1).
Main diagnostic algorithm includes thyroid ultrasonography and biopsy from suspicious nodules for thyroid malignancies. Nodules greater than 1 cm. or having suspicious features such as solid appearance, hypoechogenicity, microcalcifications on ultrasound are candidates for fine needle aspiration biopsy (4). For our patient need for tracheotomy and unresectability of the tumor decided us to make an open biopsy during operation. Since exact diagnosis is made by pathology; histological and immunohistochemical features are important for each thyroid malignancy.
MTC has unique features for diagnosis both biochemically and pathologically. Since tumor cells secrete calcitonin; serum calcitonin level is a reliable marker for diagnosis and recurrences. When basal level of calcitonin is >100 pg/mL, it is considered to be 100% predictive for diagnosis (5). Calcitonin is also used immunohistochemically; only occasionally very few or focal groups of tumor cells show calcitonin positivity (6). Amyloid deposition is seen in about 60-70% of cases (3). Since MTC is a NET; neuroendocrine markers such as neuron specific enolase, synaptophysin, chromagranin A are also positive immunohistochemically. Follicular cell marker thyroglobulin is negative in tumor cells (5).
Since the exact NET of thyroid is MTC, when tumor cells show negative reactivity to calcitonin, but stain positively with neuroendocrine markers such as Chromogranin A and synaptophysin, differential diagnosis is conflicting. Near MTC, paragangliomas, primary oat (small) cell carcinoma of thyroid, metastatic NETs, hyalinising trabecular tumors can show neuroendocrine properties (6). MTC may be indistinguishable from others morphologically; immunohistochemistry, electron microscopy, in situ hybridization may be mandatory to confirm diagnosis (6). Primary oat cell carcinoma of thyroid gland has also calcitonin negativity. Although our case couldn’t be strictly excluded for diagnosis of small cell thyroid carcinoma; these tumors mostly show cytoplasmic CEA positivity (7) which was absent for our case. Also tumor cells’ size were larger for an oat cell carcinoma in our case. Paragangliomas are NETs derived from neural crest paraganglia of autonomic system. They can rarely be seen in thyroid gland (8). Immunohistochemically they show calcitonin negativity, chromogranin A and synaptophysin positivity like our case. For our patient since there was no S100 protein positivity which shows sustentacular cells of paragangliomas at the periphery of the tumor (8) diagnosis of a paraganglioma was excluded. Metastatic NETs from other organs to thyroid can also show neuroendocrine properties. They are mostly calcitonin negative like in our case (9). Since the thyroid mass was much more bigger than intra-abdominal metastases and thoracic examination was normal; thyroid gland was regarded as the primary site. Hyalinising trabecular tumors of thyroid also have neuroendocrine properties, but they have constantly thyroglobulin positivity which was absent in our case (6).
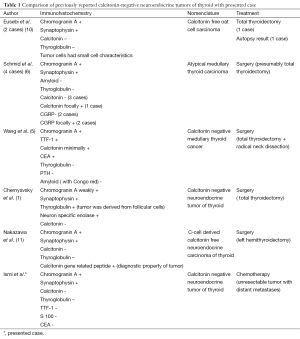
In 1990, Eusebi et al. used the term “calcitonin-free oat cell carcinoma of thyroid’’, for two cases showing calcitonin and thyroglobulin negativity with neuroendocrine properties and similar morphological features like small cell carcinoma of the lung (10). For Schmid et al. the term “atypical MTC” was suitable for calcitonin negative, neuroendocrine markers positive thyroid tumors. They concluded that although such tumors involve an obvious dilemma; calcitonin negativity is the atypical form of a MTC (6). In 2008, Wang et al. published another case of calcitonin negative MTC and they reviewed similar cases. They used the term MTC, since tumor cells were positive for CEA although minimally positive for calcitonin and negative for amyloid deposition with congo red staining (5). In 2011, Chernyavsky et al. showed a case with calcitonin negativity and NSE, synaptophysin and thyroglobulin positivity. Since thyroglobulin was positive, tumor was arising from true follicular cells rather than parafollicular cells. They used the term “calcitonin negative NET (CNNET)” for such cases when calcitonin immunoreactivity was negative, rather than the term MTC (1). For our case; patient serum calcitonin was in normal limits and immunohistochemically tumor was negative for calcitonin, thyroglobulin and amyloid staining. Since MTC cells are mostly amyloid positive, and diagnosis of all MTCs must be confirmed by calcitonin immunoreactivity (3) we prefer to use the term “calcitonin negative NET of thyroid’’ like Chernyavsky et al. (1) for our case. Recently, Nakazawa et al. used the term “C-cell derived calcitonin free neuroendocrine carcinoma of thyroid’’. They published a case with similar immunohistochemical features. Tumor cells were negative for calcitonin and positive for neuroendocrine markers. The diagnosis of atypical MTC was made by immunohistochemically with calcitonin gene related peptide positivity (11). Reported cases for calcitonin negative neuroendocrine marker positive tumors were summarized in Table 1.
Full table
NETs are wide spectrum tumors with different prognosis; paragangliomas are benign whereas Merkel cell carcinomas are aggressive malignant tumors in this group, and MTCs have prognostic properties between these two (2). Number of CNNETs are really rare and little is known about their prognosis. There are immunohistochemically differences even among these tumors. All of the presented cases were thyroglobulin negative immunohistochemically except Chernyavsky et al. In their case tumor cells showed thyroglobulin positivity regarding the follicular cell derived NET (1). Nakazawa et al. diagnosed their patient as calcitonin free NET of thyroid with CGRP immunoreactivity which was negative for cases of Schmid et al. (6,11). Since CNNETs don’t have unique pathological properties, they don’t have unique kind of prognostic features, too. Unlike MTC, they have no serum markers like calcitonin for early diagnosis of recurrences which makes the follow-up more difficult (5). For MTCs poor prognostic factors include presence of distant or lymph node metastases, tumor size >4 cm, advanced age, highly elevated preoperatively calcitonin levels. Undifferentiated tumor cells losing calcitonin secreting capacity and tumor cells <25% staining with calcitonin are also speculated to be poor prognostic factors (5). According to our knowledge; among previously reported cases our case had the most bulky and disseminated tumor which gave an impression of anaplastic or insular carcinoma with rapidly growing tumor volume and intra-abdominal metastases (Table 1). Insular carcinoma and anaplastic carcinoma diagnosis were excluded by means of neuroendocrine properties of our case (12).
Main treatment of MTC is surgery, since tumor cells aren’t sensitive to radioactive iodine uptake (3). For CNNET, most of the published cases were also treated surgically for a local disease (Table 1). Since our case was metastatic during diagnosis chemotherapy was the treatment of choice.
As a conclusion, CNNET are a group of thyroid malignancies with calcitonin negativity and neuroendocrine marker positivity immunohistochemically. Few cases are published in the literature precluding prediction of their biological behaviour and prognosis. They are mostly localized in thyroid gland treated surgically, but occasionally they can have huge tumor volume with distant metastases mimicking anaplastic carcinoma like in our case.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chernyavsky VS, Farghani S, Davidov T, et al. Calcitonin-negative neuroendocrine tumor of the thyroid: a distinct clinical entity. Thyroid 2011;21:193-6. [PubMed]
- Jacobs MA, Weinstein S, Hope TA, et al. Neuroendocrine tumors: beyond the abdomen. J Comput Assist Tomogr 2014;38:898-914. [PubMed]
- Pacini F, Castagna MG, Cipri C, et al. Medullary thyroid carcinoma. Clin Oncol (R Coll Radiol) 2010;22:475-85. [PubMed]
- Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am 2010;43:229-38. [PubMed]
- Wang TS, Ocal IT, Sosa JA, et al. Medullary thyroid carcinoma without marked elevation of calcitonin: a diagnostic and surveillance dilemma. Thyroid 2008;18:889-94. [PubMed]
- Schmid KW, Ensinger C. "Atypical" medullary thyroid carcinoma with little or no calcitonin expression. Virchows Arch 1998;433:209-15. [PubMed]
- Cruz J, Eloy C, Aragüés JM, et al. Small-cell (basaloid) thyroid carcinoma: a neoplasm with a solid cell nest histogenesis? Int J Surg Pathol 2011;19:620-6. [PubMed]
- Yu BH, Sheng WQ, Wang J. Primary paraganglioma of thyroid gland: a clinicopathologic and immunohistochemical analysis of three cases with a review of the literature. Head Neck Pathol 2013;7:373-80. [PubMed]
- Sivrikoz E, Ozbey NC, Kaya B, et al. Neuroendocrine tumors presenting with thyroid gland metastasis: a case series. J Med Case Rep 2012;6:73. [PubMed]
- Eusebi V, Damiani S, Riva C, et al. Calcitonin free oat-cell carcinoma of the thyroid gland. Virchows Arch A Pathol Anat Histopathol 1990;417:267-71. [PubMed]
- Nakazawa T, Cameselle-Teijeiro J, Vinagre J, et al. C-cell-derived calcitonin-free neuroendocrine carcinoma of the thyroid: the diagnostic importance of CGRP immunoreactivity. Int J Surg Pathol 2014;22:530-5. [PubMed]
- Cornetta AJ, Burchard AE, Pribitkin EA, et al. Insular carcinoma of the thyroid. Ear Nose Throat J 2003;82:384-6, 388-9. [PubMed]