The current status of intraoperative iPTH assay in surgery for primary hyperparathyroidism
Introduction
Since the time when the first parathyroidectomy was performed by Dr. Felix Mandl in 1925, the procedure of bilateral neck exploration done by an experienced parathyroid surgeon has been for many years the gold standard in parathyroid surgery, allowing for achieving cure rates exceeding 95% of patients with primary hyperparathyroidism (1,2). Nevertheless, the recently developed modern imaging techniques allowing for preoperative localization of diseased parathyroid gland, such as parathyroid scintigraphy or high-resolution ultrasonography, followed by developing a method of intraoperative quality control of surgical treatment based on intraoperative serum intact parathyroid hormone (iPTH) assay, became the milestones in forming the idea of minimally invasive parathyroidectomy, focusing on using a small incision to resect a solitary image-indexed parathyroid adenoma without a necessity of intraoperative identification and evaluation of the remaining parathyroids (3).
According to the literature published to date, minimally invasive parathyroidectomy has proven to be equally effective in restoring normocalcemia as bilateral neck exploration and is associated with a minimal risk of complications (4-6). A fundamental advantage of minimally invasive parathyroidectomy, in addition to better cosmetics effects and lesser pain is a significant decrease of the percentage of postoperative transient hypoparathyroidism to approximately 5% as compared to approximately 15-25% after bilateral neck exploration, as well as complete elimination of the risk of permanent hypoparathyroidism. This phenomenon is a consequence of preserving intact the blood supply of normal parathyroids, which have not been exposed in the course of minimally invasive parathyroidectomy. In turn, a lower rate of transient hypocalcemia after minimally invasive parathyroidectomy is associated with significantly decreased requirements for calcium and vitamin D3 preparations and a shorter hospitalization (7).
Historical perspective
In 1988, Nussbaum et al. modified the original immunoradiometric assay by increasing the temperature of incubation and employing a kinetic enhancer; the above changes decreased the turnover time to approximately 15 minutes (8). In this initial report, the first use of iPTH monitoring during parathyroidectomy was described, although the patients in this series underwent bilateral neck exploration and iPTH was measured postoperatively. Although the reporting of this experience appeared to be of clinical interest, it was not readily accepted as an alternative to the existing practice of highly successful conventional bilateral neck exploration. In 1990, Chapuis et al. from Paris reported in French their series of 13 patients in whom the iPTH dropped above 70% in 20 minutes after parathyroidectomy by using the immunoradiometric assay for intraoperative iPTH measurement (9). Utilizing a modification of the technique described by Nussbaum, Irvin was able to demonstrate a rapid decline in parathyroid hormone levels measured intraoperatively following removal of the second parathyroid adenoma. In 1991, Irvin et al. described for the first time a series of 21 patients who had their parathyroidectomy guided exclusively by intraoperative iPTH assay using an immunoradiometric method (10). With George Irvin’s help, in 1996, this rapid assay method was developed further to an immunochemiluminescence method, and the “quick” iPTH assay became commercially available for intraoperative use, which is still the methodology used today (11). Currently, the majority of high-volume parathyroid surgeons utilize this technique to guide parathyroidectomy in patients with a sporadic primary hyperparathyroidism (12-18).
Areas of application of intraoperative iPTH monitoring
The intraoperative iPTH assay can be utilized in three discreet modes of application:
- To guide surgical decisions during parathyroidectomy in one of the following clinical contexts:
- To confirm complete removal of all hyperfunctioning parathyroid tissue, which allows for termination of surgery with confidence that the hyperparathyroid state has been successfully corrected (12-16);
- To identify patients with additional hyperfunctioning parathyroid tissue following the incomplete removal of diseased parathyroid/s, which necessitates extended neck exploration in order to minimize the risk of operative failure (17,18);
- To differentiate parathyroid from non-parathyroid tissue by iPTH measurement in the fine-needle aspiration washout (19-21);
- To lateralize the side of the neck harboring hyperfunctioning parathyroid tissue by determination of jugular venous gradient in patients with negative or discordant preoperative imaging studies, in order to increase the number of patients eligible for unilateral neck exploration (22,23).
Protocol of intraoperative iPTH assay
A peripheral vein access is most commonly used for collection of blood samples. This access should be kept open with saline infusion throughout the procedure, and an intravenous extension is used to give the anesthesiologist access to the tubing for blood collection at times requested by the surgeon. It is extremely important to instruct the anesthesia team about discarding 10 mL of blood with saline to avoid sample dilution, potentially leading to falsely lower iPTH values. Totally 3 mL of blood are collected for iPTH measurement and are placed in an ethylenediaminetetraacetic acid (EDTA) coated tube at specific time-points and immediately centrifuged. To achieve reliable results, it is strongly recommended to follow the strict protocol of blood testing at specific time-points during parathyroidectomy, which allows for understanding the hormone dynamics during the operation. The following time-points of blood sampling for iPTH are most commonly used: (I) in the operating room before the skin incision is made (pre-incision baseline); (II) just before the blood supply to the suspicious parathyroid gland is ligated (pre-excision baseline); (III) at 10 minutes (10 minutes post-excision); and (IV) occasionally at 20 minutes after excision (20 minutes post-excision) of the suspected abnormal gland (Figure 1). An intraoperative iPTH drop of more than 50% from the highest either pre-incision or pre-excision baseline at 10 minutes post-excision is highly accurate in predicting postoperative normal or low serum calcium values (Miami criterion). The iPTH assay total turnaround time may vary from 8 to 15 minutes depending on the laboratory. During this waiting time, the surgeon can close the incision, but any manipulation of the remaining parathyroids should be avoided in order to minimize the chance of falsely elevating iPTH levels resulting in a delay in hormone drop. If the assumed criterion is not met at 10 minutes post-excision, the extended neck exploration is undertaken and the protocol for blood sampling is repeated for each additional excised suspicious parathyroid gland until all hypersecreting parathyroid tissue is removed, which is confirmed by meeting the criterion. In cases approaching but not meeting the assumed criterion of an iPTH drop at 10 minutes post-excision, some surgeons recommend obtaining an additional 20-minute post-excision sample for iPTH measurement in order to rule out the false negative result of the testing. However, such an approach is not uniformly agreed upon and some data suggest that extended neck exploration should be rather attempted instead.
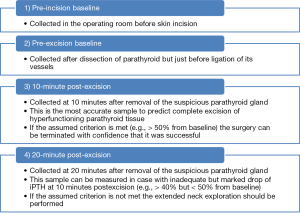
Intraoperative iPTH assay criteria for prognostication of success
The issue of appropriate patient selection plays a fundamental role in achieving a high success rate of minimally invasive parathyroidectomy approaching 100%. To achieve a high success rate of parathyroidectomy, the surgeon needs to be aware of intraoperative hormone dynamics during the case and carefully choose the protocol and interpretation criteria that best fit the individual practice. Understanding the nuances of intraoperative iPTH monitoring allows the surgeon for achieving intraoperative confidence in predicting operative success and preventing failure in cases of unsuspected multiglandular disease, while safely limiting neck exploration in the majority of patients with sporadic primary hyperparathyroidism. When concordant results of functional imaging (e.g., sestamibi scanning) and ultrasound performed by an experienced investigator are obtained, minimally invasive parathyroidectomy can be safely recommended (6,24-26). The prevalence of multiglandular parathyroid disease among patients with primary hyperparathyroidism and concordant imaging tests varies from 1% to 3.5% (26,27). Thus, when preoperative localization with sestamibi and ultrasound is concordant for single-gland disease, the use of intraoperative iPTH monitoring is of little value. However, if preoperative localization with sestamibi and ultrasound is not concordant and the surgeon wishes to perform a minimally invasive “selective” operation, the use of intraoperative iPTH monitoring is recommended, as the prevalence of multiglandular disease in this subgroup of patients with primary hyperparathyroidism approaches 17% (25,27,28). Similarly, the use of intraoperative iPTH monitoring is recommended for patients undergoing selective parathyroidectomy on the basis of a single preoperative localization study (27,28).
On the other hand, the accuracy of intraoperative iPTH monitoring in the detection of patients with multiglandular disease is highly dependent on the criteria applied. Few studies have shown that the Miami criterion followed by the Vienna criterion is the best balanced among other criteria, with the highest accuracy in intraoperative prediction of cure (26,29,30). However, the Rome criterion followed by the Halle criterion is most useful in intraoperative detection of multiglandular disease (26,30,31). Nevertheless, their application in patients qualified for minimally invasive parathyroidectomy with concordant results of sestamibi scanning and ultrasound of the neck would result in a significantly higher number of negative conversions to bilateral neck explorations and only a marginal improvement in the success rate of primary operations (26). Thus, the accuracy of intraoperative iPTH monitoring is highly dependent on the criteria used by the surgeon to predict the outcome of parathyroid surgery. The most common criteria used for prognostication of the outcome of parathyroid surgery and their predictive values are summarized in Table 1 (26).

Full table
Value of intraoperative iPTH monitoring in predicting recurrence
Schneider et al. reported on long-term results of 1,368 parathyroid operations for primary hyperparathyroidism with intraoperative iPTH monitoring, including 1,006 minimally invasive parathyroidectomies and 380 conventional parathyroidectomies. There were no differences in recurrence between the minimally invasive and conventionally operated groups (2.5% vs. 2.1%; P=0.68), and the operative approach did not independently predict recurrent disease in the multivariate analysis. However, the percentage decrease in intraoperative iPTH was protective against recurrence for both the entire cohort (hazard ratio =0.96; 95% confidence interval, 0.93-0.99; P=0.03) and the minimally invasive subset. In addition, a higher postoperative iPTH levels also independently predicted disease recurrence. Thus, the percentage decrease in intraoperative iPTH is one of many adjuncts the surgeon can use to determine which patients are best served by bilateral exploration, whereas the postoperative iPTH can guide follow-up after parathyroidectomy (32).
Wachtel et al. analyzed 2,185 subjects undergoing parathyroidectomy with intraoperative iPTH monitoring and noted that 5.0% (n=110) experienced intraoperative failure (defined as failure to decrease iPTH intraoperatively by ≥50% and into the normal range). The intraoperative failure group had more multiglandular disease (35.2% vs. 16.6%, P<0.001) and smaller glands (13 vs. 15 mm, P=0.048) compared to the patients who experienced intraoperative success. On multivariate analysis, post-excision iPTH level was statistically, but not clinically, significantly associated with intraoperative failure (odds ratio =1.0; 95% confidence interval, 1.000-1.003). Persistent hyperparathyroidism was identified in 2.5% (n=15) of 592 patients with ≥6-month follow-up. Median intraoperative iPTH decrease was lower in patients with persistent hyperparathyroidism (67.1% vs. 85.8%, P<0.001). Thus, the authors concluded that intraoperative failure was associated with higher rates of multiglandular disease and smaller parathyroid glands. In addition, patients with persistent disease had significantly lower decreases in intraoperative iPTH values, but one-half of patients who experienced failure by intraoperative iPTH assay criterion were eucalcemic 6-month postoperatively (33). This observation was also confirmed by Wharry et al., who analyzed 1,108 initial parathyroid operations for sporadic primary hyperparathyroidism using intraoperative iPTH monitoring and reported that a final intraoperative iPTH level that was within the normal range and dropped by >50% from baseline was a strong predictor of operative success. Long-term recurrence was more likely in patients with a final intraoperative iPTH level of 41-65 pg/mL than with a level ≤40 pg/mL (1.2% vs. 0%; P=0.016). Hence, patients with a final intraoperative iPTH level between 41-65 pg/mL should be followed up beyond 6 months for long-term recurrence (34).
Cost-effectiveness of intraoperative iPTH monitoring
The added value of intraoperative iPTH monitoring remains controversial, because its ability to prevent failure of parathyroidectomy due to unrecognized multiple gland disease must be balanced against assay-related costs. Morris et al. performed a literature review focused on this issue and identified 17 studies involving 4,280 unique patients, permitting estimation of base case costs and probabilities using a decision tree and cost analysis model (35). The base case assumption was that in well-localized primary hyperparathyroidism, intraoperative iPTH monitoring would increase the success rate of minimally invasive parathyroidectomy from 96.3% to 98.8%. The cost of intraoperative iPTH varied with operating room time used. Intraoperative iPTH monitoring reduced overall treatment costs only when total assay-related costs fell below $110 per case. Inaccurate localization and high reoperation cost both independently increased the value of intraoperative iPTH monitoring. The intraoperative iPTH strategy was cost-saving when the rate of unrecognized multiglandular disease exceeded 6% or if the cost of reoperation exceeded $12,000 (compared with initial minimally invasive parathyroidectomy cost of $3,733). Setting the positive predictive value (PPV) of intraoperative iPTH monitoring at 100% and reducing the false-negative rate to 0% did not substantially alter these findings. The authors concluded that institution-specific factors influenced the value of intraoperative iPTH monitoring. In the analyzed model, intraoperative iPTH monitoring increased the cure rate marginally, while incurring approximately 4% of additional cost (35). One should also take into consideration that advantages and disadvantages of the variety of existing intraoperative iPTH monitoring success criteria are confusing and their assessment is often contradictory. Hence, particularly with respect to cost-benefit aspects, the standard application of this method of intraoperative quality control even in conventional open parathyroidectomy remains a matter of controversy (36). However, the use of intraoperative iPTH monitoring compensates for its cost by shortening operative time and obviating the need for frozen sections. To decrease the cost of this intraoperative adjunct, some hospitals place the assay cart at the central laboratory, where the system can be used for other purposes and the technician does not need to be relocated to the operative room. This surgical adjunct is most helpful in reducing operative times when used as a point-of-care system in close proximity to the operating room, where PTH levels can be reported as soon as possible, allowing for real-time operative decisions based on iPTH dynamics (37).
The European Society of Endocrine Surgeons (ESES) recommended the use of intraoperative iPTH monitoring for patients undergoing “targeted” parathyroidectomy on the basis of a single preoperative localization study. If preoperative localization with sestamibi and ultrasound is not concordant and the surgeon wishes to perform a minimally invasive “targeted procedure”, the use of intraoperative iPTH monitoring is recommended. When preoperative localization with sestamibi and ultrasound is concordant for single-gland disease, the use of this adjunct is of little value. In addition, the use of intraoperative iPTH can be recommended in reoperative parathyroidectomy to lateralize hyperfunctioning parathyroid tissue (internal jugular vein/s sampling) when preoperative localization is uncertain, or to predict cure and reduce the need for continued exploration in the scarred neck (28).
Other applications for intraoperative iPTH monitoring
Intraoperative iPTH assay can be utilized to differentiate between parathyroid and non-parathyroid tissue, such as thyroid nodules and lymph nodes, with a specificity of 100% by iPTH measurement in the fine-needle aspiration washout (19-21). The aspirated content in the needle is diluted with 1 mL of saline solution, centrifuged, and the supernatant is used for iPTH measurement. This technique is faster than frozen section if the quick assay is used as a point-of-care system, which can be very helpful when gland identification is difficult, e.g., in the case of an intrathyroidal parathyroid or a lesion that could be a thyroid nodule versus a subcapsular parathyroid gland (37).
In addition, intraoperative iPTH measurement can be used to lateralize the side of the neck harboring hyperfunctioning parathyroid tissue by determination of jugular venous gradient in patients with negative or discordant preoperative imaging studies, in order to increase the number of patients eligible for unilateral neck exploration (22,23). In this technique, which is positive in 70% to 81% of cases, 3 mL of whole blood is collected under ultrasound guidance from the most caudal portion in the neck of both internal jugular veins, just before skin incision. Intact PTH levels are then measured in both jugular vein samples, as well as in the initially collected sample from the peripheral access (37). The unilateral neck exploration is undertaken on the side of the neck indexed by the highest iPTH value and terminated after successful removal of a hyperfunctioning parathyroid gland using intraoperative iPTH monitoring to assure cure from hyperparathyroid state.
Conclusions
Minimally invasive parathyroidectomy guided by intraoperative PTH monitoring is widely accepted among parathyroid surgeons for the treatment of sporadic primary hyperparathyroidism. This intraoperative adjunct warrants the operation to be a safe, highly successful, less invasive procedure, and is associated with a lower prevalence of morbidity than bilateral neck exploration. Surgical awareness of hormone dynamics during parathyroidectomy and adherence to the sampling protocol and interpretation criteria that best fit the individual practice are crucial in achieving intraoperative confidence in predicting operative success and preventing failure in cases of unsuspected multiglandular disease, while safely limiting neck exploration in the majority of patients with sporadic primary hyperparathyroidism.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Low RA, Katz AD. Parathyroidectomy via bilateral cervical exploration: a retrospective review of 866 cases. Head Neck 1998;20:583-7. [PubMed]
- Allendorf J, DiGorgi M, Spanknebel K, et al. 1112 consecutive bilateral neck explorations for primary hyperparathyroidism. World J Surg 2007;31:2075-80. [PubMed]
- Chen H, Mack E, Starling JR. A comprehensive evaluation of perioperative adjuncts during minimally invasive parathyroidectomy: which is most reliable? Ann Surg 2005;242:375-80; discussion 380-3. [PubMed]
- Miccoli P, Berti P, Materazzi G, et al. Results of video-assisted parathyroidectomy: single institution's six-year experience. World J Surg 2004;28:1216-8. [PubMed]
- Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg 2002;235:665-70; discussion 670-2. [PubMed]
- Barczyński M, Cichoń S, Konturek A, et al. Minimally invasive video-assisted parathyroidectomy versus open minimally invasive parathyroidectomy for a solitary parathyroid adenoma: a prospective, randomized, blinded trial. World J Surg 2006;30:721-31. [PubMed]
- Bergenfelz A, Kanngiesser V, Zielke A, et al. Conventional bilateral cervical exploration versus open minimally invasive parathyroidectomy under local anaesthesia for primary hyperparathyroidism. Br J Surg 2005;92:190-7. [PubMed]
- Nussbaum SR, Thompson AR, Hutcheson KA, et al. Intraoperative measurement of parathyroid hormone in the surgical management of hyperparathyroidism. Surgery 1988;104:1121-7. [PubMed]
- Chapuis Y, Fulla Y, Icard P, et al. Peroperative assay of active parathormone 1-84 in surgery of primary hyperparathyroidism. Presse Med 1990;19:1461-2. [PubMed]
- Irvin GL 3rd, Dembrow VD, Prudhomme DL. Operative monitoring of parathyroid gland hyperfunction. Am J Surg 1991;162:299-302. [PubMed]
- Boggs JE, Irvin GL 3rd, Molinari AS, et al. Intraoperative parathyroid hormone monitoring as an adjunct to parathyroidectomy. Surgery 1996;120:954-8. [PubMed]
- Irvin GL 3rd, Carneiro DM, Solorzano CC. Progress in the operative management of sporadic primary hyperparathyroidism over 34 years. Ann Surg 2004;239:704-8; discussion 708-11. [PubMed]
- Irvin GL 3rd, Solorzano CC, Carneiro DM. Quick intraoperative parathyroid hormone assay: surgical adjunct to allow limited parathyroidectomy, improve success rate, and predict outcome. World J Surg 2004;28:1287-92. [PubMed]
- Grant CS, Thompson G, Farley D, et al. Primary hyperparathyroidism surgical management since the introduction of minimally invasive parathyroidectomy: Mayo Clinic experience. Arch Surg 2005;140:472-8; discussion 478-9. [PubMed]
- Westerdahl J, Bergenfelz A. Sestamibi scan-directed parathyroid surgery: potentially high failure rate without measurement of intraoperative parathyroid hormone. World J Surg 2004;28:1132-8. [PubMed]
- Chen H, Pruhs Z, Starling JR, et al. Intraoperative parathyroid hormone testing improves cure rates in patients undergoing minimally invasive parathyroidectomy. Surgery 2005;138:583-7; discussion 587-90. [PubMed]
- Cayo AK, Sippel RS, Schaefer S, et al. Utility of intraoperative PTH for primary hyperparathyroidism due to multigland disease. Ann Surg Oncol 2009;16:3450-4. [PubMed]
- Hughes DT, Miller BS, Doherty GM, et al. Intraoperative parathyroid hormone monitoring in patients with recognized multiglandular primary hyperparathyroidism. World J Surg 2011;35:336-41. [PubMed]
- Chan RK, Ibrahim SI, Pil P, et al. Validation of a method to replace frozen section during parathyroid exploration by using the rapid parathyroid hormone assay on parathyroid aspirates. Arch Surg 2005;140:371-3. [PubMed]
- Barczynski M, Golkowski F, Konturek A, et al. Technetium-99m-sestamibi subtraction scintigraphy vs. ultrasonography combined with a rapid parathyroid hormone assay in parathyroid aspirates in preoperative localization of parathyroid adenomas and in directing surgical approach. Clin Endocrinol (Oxf) 2006;65:106-13. [PubMed]
- James BC, Nagar S, Tracy M, et al. A novel, ultrarapid parathyroid hormone assay to distinguish parathyroid from nonparathyroid tissue. Surgery 2014;156:1638-43. [PubMed]
- Lew JI, Solorzano CC, Montano RE, et al. Role of intraoperative parathormone monitoring during parathyroidectomy in patients with discordant localization studies. Surgery 2008;144:299-306. [PubMed]
- Barczynski M, Konturek A, Hubalewska-Dydejczyk A, et al. Utility of intraoperative bilateral internal jugular venous sampling with rapid parathyroid hormone testing in guiding patients with a negative sestamibi scan for minimally invasive parathyroidectomy--a randomized controlled trial. Langenbecks Arch Surg 2009;394:827-35. [PubMed]
- Mihai R, Barczynski M, Iacobone M, et al. Surgical strategy for sporadic primary hyperparathyroidism an evidence-based approach to surgical strategy, patient selection, surgical access, and reoperations. Langenbecks Arch Surg 2009;394:785-98. [PubMed]
- Bergenfelz AO, Hellman P, Harrison B, et al. Positional statement of the European Society of Endocrine Surgeons (ESES) on modern techniques in pHPT surgery. Langenbecks Arch Surg 2009;394:761-4. [PubMed]
- Barczynski M, Konturek A, Hubalewska-Dydejczyk A, et al. Evaluation of Halle, Miami, Rome, and Vienna intraoperative iPTH assay criteria in guiding minimally invasive parathyroidectomy. Langenbecks Arch Surg 2009;394:843-9. [PubMed]
- Barczynski M, Konturek A, Cichon S, et al. Intraoperative parathyroid hormone assay improves outcomes of minimally invasive parathyroidectomy mainly in patients with a presumed solitary parathyroid adenoma and missing concordance of preoperative imaging. Clin Endocrinol (Oxf) 2007;66:878-85. [PubMed]
- Harrison BJ, Triponez F. Intraoperative adjuncts in surgery for primary hyperparathyroidism. Langenbecks Arch Surg 2009;394:799-809. [PubMed]
- Carneiro DM, Solorzano CC, Nader MC, et al. Comparison of intraoperative iPTH assay (QPTH) criteria in guiding parathyroidectomy: which criterion is the most accurate? Surgery 2003;134:973-9; discussion 979-81. [PubMed]
- Riss P, Kaczirek K, Heinz G, et al. A "defined baseline" in PTH monitoring increases surgical success in patients with multiple gland disease. Surgery 2007;142:398-404. [PubMed]
- Lombardi CP, Raffaelli M, Traini E, et al. Intraoperative PTH monitoring during parathyroidectomy: the need for stricter criteria to detect multiglandular disease. Langenbecks Arch Surg 2008;393:639-45. [PubMed]
- Schneider DF, Mazeh H, Chen H, et al. Predictors of recurrence in primary hyperparathyroidism: an analysis of 1386 cases. Ann Surg 2014;259:563-8. [PubMed]
- Wachtel H, Cerullo I, Bartlett EK, et al. What Can We Learn from Intraoperative Parathyroid Hormone Levels that Do Not Drop Appropriately? Ann Surg Oncol 2014. [Epub ahead of print]. [PubMed]
- Wharry LI, Yip L, Armstrong MJ, et al. The final intraoperative parathyroid hormone level: how low should it go? World J Surg 2014;38:558-63. [PubMed]
- Morris LF, Zanocco K, Ituarte PH, et al. The value of intraoperative parathyroid hormone monitoring in localized primary hyperparathyroidism: a cost analysis. Ann Surg Oncol 2010;17:679-85. [PubMed]
- Lorenz K, Dralle H. Intraoperative parathyroid hormone determination for primary hyperparathyroidism. Chirurg 2010;81:636-638-42. [PubMed]
- Carneiro-Pla D, Pellitteri PK. Intraoperative PTH monitoring during parathyroid surgery. In: Randolph GW. eds. Surgery of the thyroid and parathyroid glands, 2nd ed. Philadelphia: Elsevier Saunders, 2013:605-12.


