Surgical outcomes of minimally invasive thyroidectomy in thyroid cancer: comparison with conventional open thyroidectomy
Introduction
Thyroid cancer is one of the most common endocrine malignancies. The incidence of thyroid cancer has been increasing steadily worldwide over the past two decades (1-4). According to the Korean cancer statistics in 2013, thyroid cancer is the most common malignancy among women (5). Differentiated thyroid carcinomas (DTCs), including papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC), are more prevalent in women than in men at a 4:1 ratio (2,6,7). Therefore, postoperative scarring after thyroidectomy is an important problem, especially for young women.
Historically, conventional open thyroidectomy (COT) is the most commonly used method for open thyroid surgery, which is still the gold standard (8). COT allows exposure of both thyroid lobes, and to perform central compartment neck dissection. However, COT normally requires a long collar incision (that is usually 5–8 cm in length), wide skin flaps on the anterior neck, and a long midline opening of the strap muscles (to provide exposure of the thyroid glands) regardless of the extent of thyroidectomy planned. This conventional approach often results in a wide neck scar and a variety of potential complications related to skin flaps and vertically opening the strap muscles. This invasive procedure, therefore, can negatively impact a patient’s quality of life (9).
This cosmetic consideration to reduce postoperative scar motivated the development of various minimally invasive thyroidectomy (MIT) techniques, even in patients with thyroid cancer. MIT is defined as thyroidectomy using smaller incision in the skin rather than incision often needed in traditional thyroidectomy. Since the first report of MIT techniques from Prof. Park in 2001 (10), MIT has been conducted at our institution with the aim of avoiding a long cervical scar. Furthermore, the recent introduction of advanced energy devices has simplified the procedures, and decreased the wound size. In short, a unilateral 2–3-cm skin incision is made along the lateral border of the strap muscles in MIT.
Therefore, the first goal of this study was to describe our current MIT technique using a unilateral small incision. The second aim was to compare perioperative and oncologic outcomes of MIT with COT in patients with DTC. We present the following article in accordance with STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-512).
Methods
Patients
This was a retrospective study using data collected prospectively from the endocrine surgery database at Yonsei University College of Medicine. We received written informed consent for the publication of patient images from the patients themselves. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the University’s Institutional Review Board (IRB No. 4-2018-0504), which waived the requirement for informed consent of clinicopathologic data due to the retrospective nature of this study. We reviewed the medical records of 3,611 consecutive DTC patients who underwent thyroid lobectomy at our department between March 2006 and November 2017. Of those, 320 patients were excluded because they were lost to follow-up or had incomplete medical records. A total of 3,291 DTC patients who underwent thyroid lobectomy were enrolled in this study. Thyroid lobectomy was performed unless the following conditions were identified by preoperative evaluation: bilateral PTC, definite extrathyroidal invasion, evidence of extensive lymph node (LN) involvement, and/or distant metastasis and personal history of radiation therapy to the head and neck. Patients were divided into two groups according to the surgical technique: 617 (18.7%) patients underwent MIT and 2,674 (81.3%) patients underwent COT. The mean follow-up period was 41.2±19.7 (range, 3–142) months. TNM stage was classified based on the 7th edition of TNM staging system.
Surgical MIT procedure
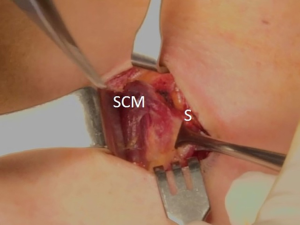
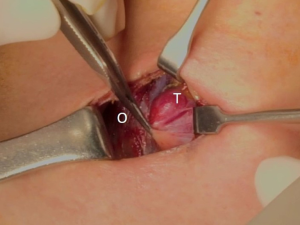
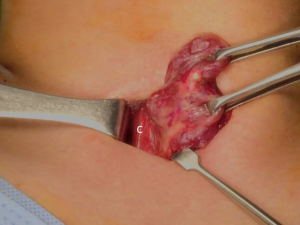
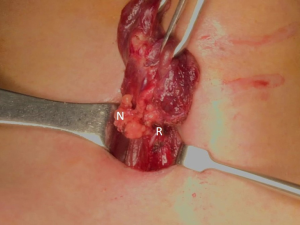
The patient was placed in the supine position on the operating table, with neck extension after general anesthesia. A 2–3-cm skin incision was made along the skin crease in the lesion side of the lower neck (Figure 1). The length of the skin incision was adjusted according to the tumor size, the size of the thyroid gland, and the presence or absence of thyroiditis. After dividing the Platysma muscle with electrocautery, skin flaps were made approximately 1 cm above and below the skin incision. The medial border of the sternocleidomastoid muscle (SCM) and the lateral border of the strap muscle were exposed using electrocautery (Figure 2). After this dissection, the omohyoid muscle could be identified, and care must be taken as the jugular vein passes under the omohyoid muscle. Along the lateral border of the strap muscles, the outside portion of the thyroid gland could be identified (Figure 3). After exposing the thyroid gland, the isthmus was completely resected over the trachea. The thyroid gland was retracted medially and inferiorly to expose its upper pole and to ligate the superior thyroidal artery and vein individually using an energetic surgical instrument. When the upper pole of thyroid gland was dissected, the whole thyroid gland could be pulled out of the skin incision (Figure 4). After confirming and preserving the inferior parathyroid gland, the recurrent laryngeal nerve was preserved, and central compartment node dissection (CCND) is performed in the same way as COT (Figure 5). Finally, Berry’s ligament was carefully divided as the specimen was obtained. The platysma and subcutaneous fatty tissues were reapproximated with interrupted 4-0 Vicryl sutures and the skin is closed using 5-0 Maxon subcuticular continuous sutures.
Postoperative management and follow-up
Postoperatively, patients were managed according to the ATA management guidelines (11). We regularly followed all the patients with neck ultrasonography and serum thyroid function testing at 6- or 12-month intervals to detect local recurrence. After his or her initial thyroid lobectomy, each patient also had a chest radiograph or a computed tomography scan to detect potential lung metastasis. Whenever recurrence was suspected in the remnant thyroid, resection bed, or LN, the diagnosis was confirmed by histologic examination using aspiration cytology or another surgery.
Outcome assessment and statistical analysis
The two groups were compared with respect to demographic and clinicopathological findings, locoregional recurrence and disease-free survival (DFS). Data were expressed as means ± standard deviations (SDs) or n (%) for descriptive statistics. All of the data were analyzed using IBM SPSS statistics software (version 23.0; SPSS Inc., Chicago IL, USA). A t-test was used for continuous variables. Pearson’s chi-square test and Fisher’s exact test were used for categorical variables. Kaplan-Meier survival analysis was used to estimate DFS. In all cases, a P values <0.05 were considered statistically significant.
Results
Baseline clinicopathologic characteristics of the study patients
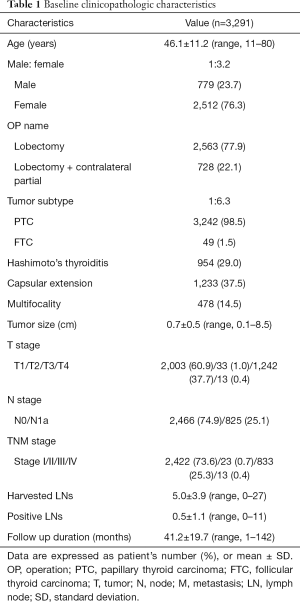
Table 1 shows the baseline clinicopathologic characteristics of 3,291 patients with DTC. The mean age of the study patients was 46.1±11.2 (range, 11–80) years. A majority of the patients (n=2,512, 76.3%) were female. There were 2,563 (77.9%) patients who underwent lobectomy, while the remaining 728 (22.1%) underwent lobectomy with contralateral partial thyroidectomy. The majority of patients (n=3,242, 98.5%) were diagnosed with PTC, while the remaining 49 (1.5%) patients were diagnosed with FTC. There were 954 (29.0%) patients with coexisting Hashimoto’s thyroiditis, defined as an elevated thyroid peroxidase antibody (TPOAb) on preoperative thyroid function tests, or histologically proven Hashimoto’s thyroiditis. Capsular extension and disease multifocality were diagnosed in 1,233 (37.5%) and 478 (14.5%) patients, respectively. The mean tumor size was 0.7±0.5 (range, 0.1–8.5) cm. The numbers of patients in each T stage were as follows: 2,003 (60.9%) in stage 1, 33 (1.0%) in stage 2, 1,242 (37.7%) in stage 3 and 13 (0.4%) in stage 4. The numbers of patients in each TNM stage were as follows: 2,422 (73.6%) in stage I, 23 (0.7%) in stage II, 833 (25.3%) in stage III and 13 (0.4%) stage IV. The mean number of harvested LNs and positive LNs were 5.0±3.9 (range, 0–27) and 0.5±1.1, respectively.
Full table
Comparison of baseline clinicopathologic characteristics and surgical outcomes between MIT and COT groups
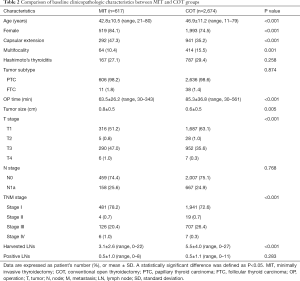
The mean age was younger in the MIT group than it was in the COT group (P<0.001). There were more female patients in the MIT group than they were in the COT group (P<0.001). Capsular extension was significantly more frequent in the MIT group than it was in the COT group (P<0.001). However, multifocality was higher in the COT group than it was in the MIT group (P=0.001). The mean tumor size was relatively larger in the MIT group than in the COT group (0.8±0.5 vs. 0.6±0.5 cm, P=0.005). The mean operation time for the MIT group was significantly shorter than that of the COT group (63.5±26.2 vs. 85.3±36.8 minutes, P<0.001). There were significantly fewer harvested LNs in the MIT group than in the COT group (3.1±2.6 vs. 5.5±4.0, P<0.001). However, there were no statistically significant differences in the coexistence of Hashimoto’s thyroiditis, tumor subtype, N stage and the number of positive LNs between the two groups (Table 2).
Full table
Table 3 compares the surgical outcomes between the two groups as well. The most common complication in both groups was transient vocal fold palsy, which was relatively more frequent in the MIT group than it was in the COT group (7.1% vs. 5.6%, P<0.001). These patients complained of hoarseness without definite intraoperative nerve injury, but achieved a satisfactory voice within 3 months. The second common complication in both groups was seroma, which was relatively more frequent in the COT group than it was in the MIT group (2.4% vs. 0.3%, P<0.001). These seromas resolved well with repeated needle aspiration. One (0.1%) patient in the MIT group and 14 (0.1%) in the COT developed minor chylous leakage due to CCND. The patients with minor chylous leakage were treated conservatively. None of the patients in the MIT group developed postoperative hematomas, while 4 in the COT group (0.1%) developed hematomas (P<0.001). The number of painkillers used after surgery was significantly lower in the MIT group than it was in the COT group (1.2±0.5 vs. 2.7±1.6, P<0.001). The length of hospital stay was shorter in the MIT than it was in the COT group (2.7±0.6 vs. 3.1±0.8 days, P<0.001). There was no statistically significant difference in the recurrence rate between the two groups (P=0.564).

Full table
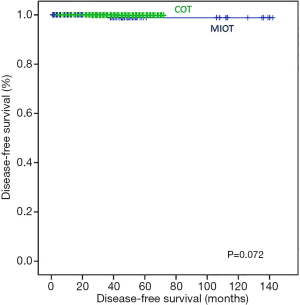
There were no mortalities during follow-up. Therefore, only DFS was compared between the two groups. In Kaplan-Meier analysis, there was no statistically significant difference in the DFS between the COT and MIT groups (log-rank P=0.072; Figure 6).
Cosmetic outcomes in the MIT group
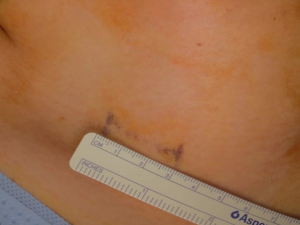
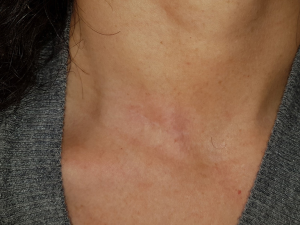
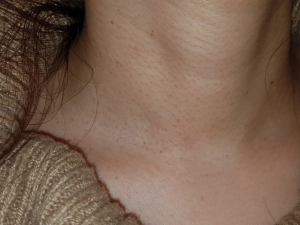
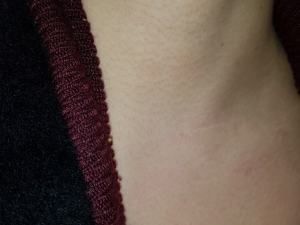
A 43-year-old woman presented with an incidentally found thyroid tumor during her annual health screening in 2017. The tumor was diagnosed as thyroid cancer by fine needle aspiration biopsy. The patient underwent MIT. The picture in Figure 7 demonstrates her wound 10 months after surgery. A 36-year-old female patient was diagnosed with thyroid cancer and underwent MIT. The picture in Figure 8 shows the wound 17 months after surgery. The next patient was a 40-year-old woman who underwent MIT after diagnosis of thyroid cancer. It is a wound after 5 years of operation (Figure 9).
Discussion
The prevalence of thyroid cancer has increased significantly in the last several decades (1,4,5). Recently, low risk intrathyroidal T1 or T2 tumors have increased due to the improvements in the diagnostic modalities (4,12,13). There is controversy as to whether or not these patients should be treated with thyroid lobectomy alone. However, several studies have found that lobectomy is equivalent to total thyroidectomy in patients with low risk intrathyroidal T1 or T2 tumors (14-16). Lobectomy can be done, when radioactive iodine treatment is not indicated. Lobectomy reduces the risk of complications such as transient/persistent postoperative hypoparathyroidism and recurrent laryngeal nerve injury.
Female patients have a higher prevalence of malignancy than do male patients. Women also tend to have greater cosmetic demands than their male counterparts (5). Therefore, various surgical methods have been developed. Transaxillary robotic thyroidectomy, which was the first performed in our hospital, is still the most widely used robotic method (17). However, robotic surgery not only requires an inevitable skin incision of about 5–7 cm near the armpit, but also a cost burden. Recently, transoral endoscopic or robotic thyroidectomy has been developed to avoid postoperative scar in the neck (18,19).
The most commonly used method for thyroid surgery is COT, which involves a 5–10-cm skin incision in the lower neck. As previously discussed, scars in the central neck are unavoidable using this method. In addition, adhesions in the skin of the neck (due to wide skin flaps), and a long midline opening of the strap muscles cause discomfort and difficulty swallowing.
Several minimally invasive open thyroidectomies have been proposed to overcome these drawbacks (10,20-22). The Sofferman Technique involves a smaller skin incision, shorter operative time, and less bleeding than does COT (20). Park et al. also reported that MIT was better than was COT with regard to the operative time, amount of intraoperative bleeding, length of hospital stay, postoperative analgesic requirements and cosmetic results (10). Although this method involved a small range of skin flaps, postoperative neck adhesion was inevitable due to the resection of the strap muscles.
The MIT surgery reported here is slightly different from the previously described method. The thyroid gland is identified along the lateral border of the strap muscles and removed without resecting the strap muscles. By using this method, we can avoid neck adhesions. We believed that this ultimately reduces patient discomfort, and increases their quality of life. However, because MIT has only been performed this way since March 2006, it is difficult to determine its long-term surgical outcomes. Only one recurrence was observed in the study population. The early surgical outcomes of MIT were similar to those of COT.
The patients in the MIT group were younger and more often female than were those in the COT group. These differences may reflect the idea that young adult women would preferentially choose the operative method that minimizes displeasing cosmetic changes. Postoperative complications were more common in MIT group than they were in the COT group (7.1% vs. 6.5%, P=0.000). Most of these complications were transient hoarseness. This may have been caused by the traction of the thyroid gland when it was pulled out of the skin incision, and by heat conduction using the energetic surgical device. Transient hoarseness was assessed clinically, not by an objective test. This may lead to an underreport of lesions. However, all patients with transient hoarseness improved within 3 months.
In order to compare the surgical outcomes between the groups, we used several perioperative parameters. These parameters included operative time, the number of painkillers used after surgery, the length of hospital stay and recurrence. The mean operative time was shorter in the MIT group than it was in the COT group. There were also significantly lower painkiller requirements in the PRA group than in the COT group. This result is potentially a result of the shorter operative time and smaller skin flaps used in the MIT group compared to those in the COT group. The same reasons may have also influenced the length of hospital stay. Although we studied short-term follow-up data, there was no difference between the two groups in the DFS (log-rank P=0.072). A pitfall of MIT was its limited collection of LNs due to the narrow space; regardless, there was no significant difference in the number of positive LNs between the two groups (0.5±1.0 vs. 0.5±1.1, P=0.283).
The indications and contraindications of MIT were previously described in the Journal of Surgical Oncology in 2008 (21). According to this article, the indications are limited to patients with the following characteristics: low to intermediate risk, well-DTC of <2 cm; thyroid nodules of <4 cm; and thyroid gland volume of <30 cc. The most important indication is for surgeons who have considerable experience with open, conventional thyroid surgery. In contrast, the contraindications for MIT include patients with history of thyroiditis, a history of previous neck surgery, head/neck irradiation, the presence of palpable lymphadenopathy, large goiters, and aggressive, high-risk, poorly-DTCs. Recent advances in imaging modalities including ultrasonography, CT, and PET/CT have improved the accuracy of the preoperative evaluation of thyroid nodules. Therefore, the extent of the operation can be planned before surgery (23). The indications for MIT in our hospital include thyroid cancer that can be resected with lobectomy, and benign tumors <4 cm in size.
This study has several limitations. The first limitation is its retrospective nature. In addition, there may have been selection bias, as all patients were collected from a single tertiary institution. Thirdly, the mean age of the MIT group was significantly younger than that of the COT group. Due to cosmetic concerns, younger patients prefer a more aesthetic surgery. Finally, the mean follow-up period was relatively short (41.2±19.7 months). This follow-up limited our ability to compare the long-term surgical outcomes between MIT and COT. Longer follow-up is necessary to predict the prognosis of patients with DTC, as it has indolent characteristics.
The most important strength of this study is that every patient was followed using a standardized protocol, including the degree of thyroid stimulating hormone (TSH) suppression and the use of imaging modalities.
Conclusions
To the best of our knowledge, this is one of few studies that have compared the surgical outcomes of MIT and COT. Our results demonstrate that MIT is technically feasible in patients with DTC. It is a valuable alternative operative technique to COT. One can expect good surgical outcomes and outstanding cosmetic effects with MIT. Regardless, further prospective studies are needed to substantiate our results.
Acknowledgments
I would like to thank all nurse helping the operations who have contributed to this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-512
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-512
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-512). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. We received written informed consent for the publication of patient images from the patients themselves. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the University’s Institutional Review Board (IRB No. 4-2018-0504), which waived the requirement for informed consent of clinicopathologic data due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cramer JD, Fu P, Harth KC, et al. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery 2010;148:1147-52; discussion 1152-3. [Crossref] [PubMed]
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317-22. [Crossref] [PubMed]
- Pellegriti G, Frasca F, Regalbuto C, et al. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J cancer epidemiol 2013;2013:965212. [Crossref] [PubMed]
- Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer 2009;115:3801-7. [Crossref] [PubMed]
- Oh CM, Won YJ, Jung KW, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat 2016;48:436-50. [Crossref] [PubMed]
- Kilfoy BA, Zheng T, Holford TR, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control 2009;20:525-31. [Crossref] [PubMed]
- Burns WR, Zeiger MA. Differentiated thyroid cancer. Semin Oncol 2010;37:557-66. [Crossref] [PubMed]
- Welbourn RB. Highlights from endocrine surgical history. World J Surg 1996;20:603-12. [Crossref] [PubMed]
- Lee MC, Park H, Lee BC, et al. Comparison of quality of life between open and endoscopic thyroidectomy for papillary thyroid cancer. Head Neck 2016;38:E827-31. [Crossref] [PubMed]
- Park CS, Chung WY, Chang HS. Minimally invasive open thyroidectomy. Surg Today 2001;31:665-9. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 2009;18:784-91. [Crossref] [PubMed]
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 2006;295:2164-7. [Crossref] [PubMed]
- Shah JP, Loree TR, Dharker D, et al. Lobectomy versus total thyroidectomy for differentiated carcinoma of the thyroid: a matched-pair analysis. Am J Surg 1993;166:331-5. [Crossref] [PubMed]
- Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery 1987;102:1088-95. [PubMed]
- Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg 1998;133:419-25. [Crossref] [PubMed]
- Kang SW, Park JH, Jeong JS, et al. Prospects of robotic thyroidectomy using a gasless, transaxillary approach for the management of thyroid carcinoma. Surg Laparosc Endosc Percutan Tech 2011;21:223-9. [Crossref] [PubMed]
- Anuwong A, Sasanakietkul T, Jitpratoom P, et al. Transoral endoscopic thyroidectomy vestibular approach (TOETVA): indications, techniques and results. Surg Endosc 2018;32:456-65. [Crossref] [PubMed]
- Kim HY, Chai YJ, Dionigi G, et al. Transoral robotic thyroidectomy: lessons learned from an initial consecutive series of 24 patients. Surg Endosc 2018;32:688-94. [Crossref] [PubMed]
- Terris DJ, Bonnett A, Gourin CG, et al. Minimally invasive thyroidectomy using the Sofferman technique. Laryngoscope 2005;115:1104-8. [Crossref] [PubMed]
- Dhiman SV, Inabnet WB. Minimally invasive surgery for thyroid diseases and thyroid cancer. J Surg Oncol 2008;97:665-8. [Crossref] [PubMed]
- Cavicchi O, Piccin O, Ceroni AR, et al. Minimally invasive nonendoscopic thyroidectomy. Otolaryngol Head Neck Surg 2006;135:744-7. [Crossref] [PubMed]
- Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol 2016;17:370-95. [Crossref] [PubMed]