Standard-dose epirubicin increases the pathological complete response rate in neoadjuvant chemotherapy for breast cancer: a multicenter retrospective study
Introduction
Breast cancer is one of the most common types of malignant tumors in females worldwide. According to the statistics on female breast cancer from the American Cancer Society, the incidence of breast cancer has increased by 0.3% annually in recent years (1). Chinese cases account for 12.2% of all newly diagnosed breast cancers and 9.6% of all deaths worldwide (2). Tremendous efforts have been made for pursing a continuous improvement of patients’ survival in patients with breast cancer, and the perspectives of disease management towards breast cancer are shifting from the traditional reduction of mortality to comprehensive management that enables curation or recurrence reduction.
Neoadjuvant chemotherapy (NAC) for breast cancer has drawn increased attention in comprehensive treatment for breast cancer. It has multiple advantages in improving surgical resection rate and breast conservation rate, reducing the probability of postoperative recurrence, and increasing the sensitivity of tumors to chemotherapy (3). Pathological complete response (pCR) following NAC is strongly associated with both breast cancer subtype and long-term survival, and it is an independent predictor of favorable clinical outcomes in all molecular subtypes (4). pCR has become an accepted evaluation index for evaluating the efficacy of NAC.
Epirubicin is a derivative from adriamycin, which is also a new generation of anthracycline antitumor drugs. Earlier NAC regimens are anthracycline-based, yet epirubicin has rapidly appeared as the first choice to treat breast cancer (5). It is noteworthy that epirubicin doses in chemotherapy regimens for Chinese patients were much lower than those in western populations for some ethnic and genetic background differences. Chinese clinicians usually reduce the epirubicin dose by 10−15% during neoadjuvant treatment for the visible side effects, including cardiotoxicity and bone marrow suppression.
It was reported that primary breast cancer tumor cells cultured in vitro with growth inhibition showed a strong dose of dependence for epirubicin (6). There were rare studies concerning the impact of epirubicin dose in NAC for Chinese patients with breast cancer. A unanimous agreement on the dose of epirubicin in this condition has not been reached so far. It is speculated the reduction of the epirubicin dose would affect the pCR rate or drug-associated toxicity. This study aims to investigate the efficacy and safety of standard-dose epirubicin in NAC for Chinese breast cancer patients retrospectively.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/gs-20-647).
Methods
Patients
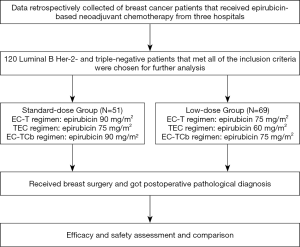
This study retrospectively collected clinical data from breast cancer patients who received epirubicin-based NAC and later surgeries from January 2015 to December 2019. Data of patients were from three independent hospitals: the First Affiliated Hospital of the University of Science and Technology of China (Anhui Provincial Hospital), the First Affiliated Hospital of Anhui Medical University, and the First Affiliated Hospital of Bengbu Medical College.
The inclusion criteria are: patients were pathologically diagnosed with Luminal B Her-2- breast cancer and triple-negative breast cancer (TNBC) by fine-needle or core-needle biopsy, respectively. The patients were clinically diagnosed as early or locally advanced breast cancer by physical examinations and imaging tests. The patients had satisfactory performance status (Eastern Cooperative Oncology Group Score ≤2). The patients underwent a subsequent surgery after NAC and got the postoperative pathological diagnosis. The patients had normal cardiac function with left ventricular ejection fraction ≥50% by echocardiography before treatment. The blood tests and cardiac examinations were recorded after chemotherapy for toxicity assessment. Patients that underwent other anti-cancer drugs or presented with other serious illness that potentially affected treatment results, such as acute myocardial infarction, cancer, pulmonary heart disease, and severe renal insufficiency were excluded.
This study was approved by the Ethics Committee of Anhui Provincial Hospital (2019-ky086). The need for written informed consent was waived by the Ethics Committee because of the retrospective nature of this study. This study was conducted following the Declaration of Helsinki (as revised in 2013).
Data collection
The clinicopathological characteristics collected are age, menopause condition, molecular subtype, clinical T stage, lymph node status, ER status, PR status, Ki-67, chemotherapy regimen, epirubicin dose, and toxicity of chemotherapy. The pCR and incidence rate of adverse events were also recorded as the primary indicators of efficacy and the index of safety.
Chemotherapy regimens
EC-T regimen: epirubicin 75–90 mg/m2 d1, cyclophosphamide 600 mg/m2 d1, every 3 weeks, 4 cycles; sequential docetaxel 80 mg/m2 d1, every 3 weeks. TEC regimen: epirubicin 60–75 mg/m2 d1, cyclophosphamide 500 mg/m2 d1, docetaxel 75 mg/m2 d1, every 3 weeks. EC-TCb regimen: epirubicin 75–90 mg/m2 d1, cyclophosphamide 600 mg/m2 d1, every 3 weeks, 4 cycles; sequential docetaxel 80 mg/m2 d1, every 3 weeks, Carboplatin AUC 5–6, every 3 weeks.
Efficacy and safety assessment
According to Miller and Payne system, pCR is defined as Grade 5: no identifiable malignant cells in sections are observed from the site of the tumor, only vascular fibroelastotic stroma remains often containing macrophages, and ductal carcinoma in situ may be present (7). Further, the assessment of treatment-related adverse events was from the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. This study recorded the grade III/IV adverse events that clinicians most concerned, which are needed to be aware and addressed adequately.
Statistical analysis
All data analysis and graph presentations were performed using SPSS 23.0 (IBM Corporation, Armonk, NY, USA) and Graphpad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) statistical software. The Chi-square test was used for rate comparison. Cox regression analysis was used for univariate and multivariate analysis. Only factors that got P<0.20 in the univariate analysis were further analyzed in multivariate analysis. The difference was statistically significant at P<0.05.
Results
Clinicopathological characteristics of patients
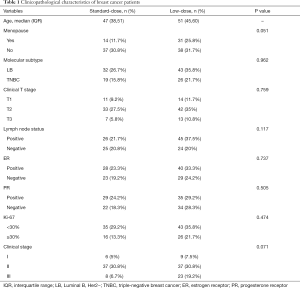
Data from 120 breast cancer patients who met the study inclusion criteria were eventually analyzed in this study. All patients underwent epirubicin-based neoadjuvant treatment. Patients were classified into two groups according to the epirubicin dose: standard-dose group (N=51) and low-dose group (N=69). A detailed study flowchart was shown (Figure 1). The median age of the standard-dose group was 47 years old, while the low-dose group was 51 years old. There is no significant difference in menopause condition, molecular subtype, clinical T stage, lymph node status, ER status, PR status, Ki-67, and clinical-stage between the two different dose groups (Table 1).
Full table
Association between clinicopathological parameters and the pCR rate
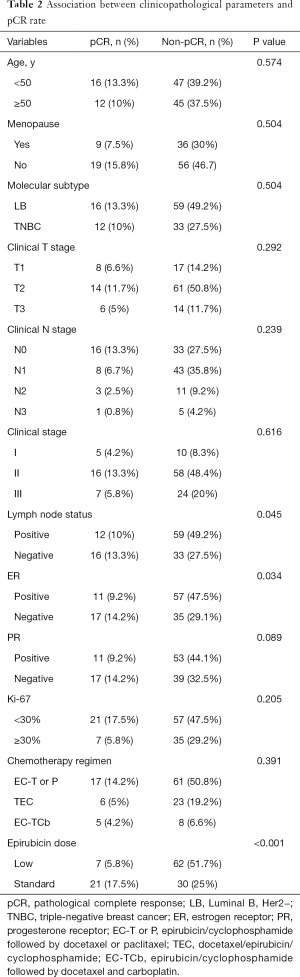
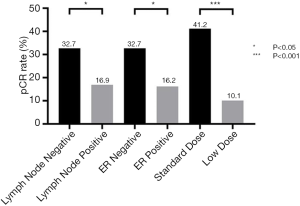
The Chi-square test showed the pCR rate difference in the clinicopathological parameter groups. The pCR rate of the standard-dose group was 41.2%, while the pCR rate of the low-dose group was 10.1% (P<0.001), and the difference was statistically significant. In the subgroup analysis, the pCR rates of ER-negative and ER-positive patients’ group were 32.7% and 16.2% (P=0.034). Meanwhile, the pCR rates of node-negative and node-positive patients were 32.7% and 16.9% (P=0.045). pCR rates showed no statistical significance between different age, menopause condition, molecular subtype, clinical T stage, clinical N stage, clinical stage, lymph node status, PR status, Ki-67, and chemotherapy groups (Table 2 and Figure 2).
Full table
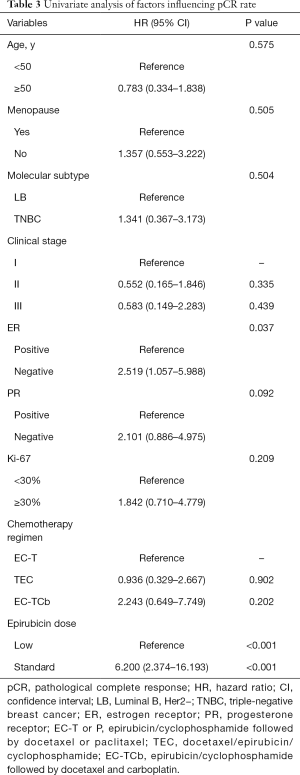
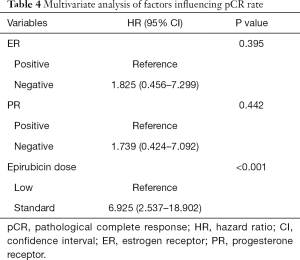
Univariate and multivariate analysis of factors influencing pCR rate
The univariate analysis showed the ER status (HR, 2.519; 95% CI, 1.057–5.988, P=0.037) and epirubicin dose (HR, 6.200; 95% CI, 2.374–16.193, P<0.001) were possibly associated with pCR rate (Table 3). Finally, three factors that got P<0.20 were further analyzed in multivariate analysis. Patients receiving standard-dose epirubicin chemotherapy (HR, 6.925; 95% CI, 2.537–18.902, P<0.001) showed more possibility to achieve pCR after NAC, it was an independent positive prognostic factor (Table 4).
Full table
Full table
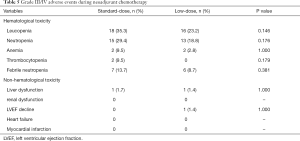
Adverse events during NAC
In terms of grade III/IV adverse events, the incidence rates of leucopenia (35.3% vs. 23.2%, P=0.146), neutropenia (29.4% vs. 18.8%, P=0.176), anemia (9.5% vs. 2.8%, P=1.000), and thrombocytopenia (9.5% vs. 0%, P=0.179) were higher in the standard-dose group than those in the low-dose group, but there was no significant difference between the two different dose groups. Notably, the incidence of febrile neutropenia, the standard-dose, and low-dose groups were 13.7% and 8.7%, but there was no statistical difference (P=0.381). The non-hematological toxicities are like the hematological toxicities (liver dysfunction, 1.7% vs. 1.4%, P=1.000) in the two different dose groups. The incidence of grade III/IV non-hematological toxicities observed was below 5%. In the low-dose group, one patient who suffered the cardiotoxicity with decreased left ventricular ejection fraction, which was not shown in patients from the standard-dose group (Table 5).
Full table
Discussion
Epirubicin plays a crucial role in neoadjuvant, adjuvant, and palliative care for breast cancer chemotherapy (8-10). Although there are other alternative medicines or treatments in NAC such as taxane-based or platinum-based chemotherapy regimens, anthracycline-based chemotherapy was the most common-used regimen in clinical practice. Chinese and western populations have different responding doses, sensitivities, and toxicity to epirubicin. Further, pharmacogenetics has influenced the pharmacokinetics and metabolism of epirubicin (11). Differences in efficacy and toxicity are because of the inter-patient variability in pharmacokinetics, not doses to body surface area (12). Racial differences in acute toxicity result in potential differential tolerance to chemotherapy, leading to compromised dose intensity in early-stage breast cancer patients who were treated with epirubicin-based chemotherapy (13). Therefore, epirubicin was empirically used in a reduced dosage for Chinese breast patients because it was dose/concentration-dependent (14).
In this study, the standard-dose group showed a higher pCR rate than the low-dose group after NAC (41.2% vs. 10.1%, P<0.001), and standard-dose epirubicin was an independent positive prognostic factor. Whether the epirubicin dose affected the treatment results is still controversial. Ackland et al. (15) investigated the dose intensity in anthracycline-based chemotherapy for metastatic breast cancer. The time to disease progression (TTP) (5.7 vs. 5.8 months, P=0.19) or overall survival (OS) (14.5 vs. 16.5 months, P=0.29) between high-dose epirubicin group and standard-dose epirubicin group had no significant differences. Also, the objective tumor response was similar (36% vs. 28%, P=0.23). The high-dose intensity group did not show superiority to epirubicin chemotherapy in disease progression, survival, or quality of life. Coombes et al. (16) found that high-dose epirubicin (HR, 0.82; 95% CI, 0.63–1.06, P=0.13) had no statistically significant effects on disease-free survival (DFS) in premenopausal patients with node-positive early breast cancer. Higher doses of epirubicin led to more adverse events while no increase in OS. Therasse et al. (17) also found that dose-intensified epirubicin did not bring a desirable therapeutic benefit in NAC for locally advanced breast cancer. However, a study from the French Adjuvant Study Group (18) proved that the high-dose epirubicin regimen in adjuvant chemotherapy led to significant benefits for node-positive breast cancer patients. The 5-year DFS was 66.3% and 54.8% (P=0.03) and the 5-year OS was 77.4% and 65.3%, respectively (P=0.007). Petit et al. (19) also found that breast cancer patients treated with high-dose anthracycline had a much better overall response rate (82.5% vs. 61.5%, P=0.038) in NAC. A conditional variable combining anthracycline dose with HER-2 status was the independent predictive factor for the overall response rate, and high-dose anthracycline and HER-2+ predicted a high overall response rate.
It was found in our study that the EC-TCb regimen (5/13, 38.46%) had a higher pCR rate trend than that in the EC-T regimen (17/78, 21.79%) or the TEC regimen (6/29, 20.69%). Different subtypes of breast cancer and NAC regimens had various pCR rates in one earlier study. von Minckwitz et al. (20) showed the pCR rates of anthracycline combined cyclophosphamide regimen and anthracycline combined cyclophosphamide following the docetaxel regimen were respectively 7.0% and 14.3% in operable breast cancer NAC (P=0.0011). Vriens et al. (21) showed the pCR rates of anthracycline combined cyclophosphamide and taxane regimen and anthracycline combined cyclophosphamide following the taxane regimen in NAC for breast cancer were respectively 16% and 21%, and the anthracycline sequential taxane regimen had higher pCR rates. The GeparSixto-GBG66 clinical trial (22) showed that the pCR rate was significantly increased by adding carboplatin from 36.9% to 43.7% in the anthracycline-combined taxane regimen for TNBC patients. Meanwhile, the CALGB 40603 clinical trial (23) got a similar conclusion that the pCR rate rose to 60% in TNBC patients because of the addition of carboplatin.
As for adverse events, we found no significant differences between the two different dose groups in terms of grade III/IV toxicity. Cardiotoxicity was observed in one patient in the low-dose group during epirubicin medication in our study. Zhou et al. (24) compared the efficacy and safety of three anthracycline-based NAC regimens in breast cancer. The percentages of patients with grade III/IV neutropenia and liver dysfunction in the epirubicin (100 mg/m2) combined cyclophosphamide group were 72.0% and 3.7%, respectively. Pizzuti et al. (25) found that breast cancer patients accepted a high-dose epirubicin-based (120 mg/m2) The NAC regimen, combined with trastuzumab. The results showed the percentages of grade III/IV neutropenia, febrile neutropenia, anemia, and thrombocytopenia were 77.8%, 20%, 2.2%, and 0%, respectively. Despite the concurrent use of trastuzumab and anthracycline, researchers did not observe any clinical cardiotoxicity. Ackland et al. (15) illustrated there was more toxicity in anthracycline-based (150 mg/m2) chemotherapy for metastatic breast cancer. The percentages of grade III/IV neutropenia, febrile neutropenia, anemia, and thrombocytopenia were 98%, 50%, 37%, and 65%, with no cases of cardiotoxicity. Therasse et al. (17) reported the percentages of grade III/IV neutropenia, febrile neutropenia, anemia, and thrombocytopenia were 78.1%, 8.4%, 50.9%, and 33.1% in locally advanced breast cancer treated with dose-intensified epirubicin (120 mg/m2) NAC regimen. Only two patients finally developed symptomatic congestive heart failure (2/224, 0.89%). The incidence of neutropenia in our study was lower than the work previously reported. It is due to the combined preventive granulocyte-colony stimulating factor (G-CSF) treatment.
This study had several limitations. First, the molecular subtype of patients in this study only covered Luminal B, Her2− breast cancer, TNBC, and uncovered Her2+ breast cancer. These two types of breast cancer have a better therapeutic effect in NAC than Luminal A breast cancer. Besides, it excluded the interference of trastuzumab. Second, the survival data was lacking because the DFS and OS are still under the long-term follow-up period. Third, this was a retrospective study, and further prospective clinical studies must enroll more patients to confirm the present findings from this study.
Conclusions
Standard-dose epirubicin increases the pCR rate in NAC for breast cancer without extra grade III/IV adverse events. It is a potential treatment choice for Chinese breast cancer patients.
Acknowledgments
Funding: This study was supported by the Key Research and Development Projects from Science and Technology Department of Anhui Province (1704a0802148, 1804h08020259), the Fundamental Research Funds for the Central Universities (WK9110000058), the Natural Science Foundation of Anhui Province (1908085MH260) and the Hefei Municipal Independent Innovation Policy “Borrowing and Transferring” Project (J2018Y01).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/gs-20-647
Data Sharing Statement: Available at http://dx.doi.org/10.21037/gs-20-647
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/gs-20-647). Dr. Pan reports grants from Fundamental Research Funds for the Central Universities, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Anhui Provincial Hospital (2019-ky086). The need for written informed consent was waived by the Ethics Committee because of the retrospective nature of this study. This study was conducted following the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69:438-51. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Zhu J, Jiao D, Guo X, et al. Predictive factors and prognostic value of pathologic complete response of ipsilateral supraclavicular lymph nodes in breast cancer after neoadjuvant chemotherapy. Ann Transl Med 2019;7:666. [Crossref] [PubMed]
- Bonnefoi H, Litière S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol 2014;25:1128-36. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Konecny G, Untch M, Slamon D, et al. Drug interactions and cytotoxic effects of paclitaxel in combination with carboplatin, epirubicin, gemcitabine or vinorelbine in breast cancer cell lines and tumor samples. Breast Cancer Res Treat 2001;67:223-33. [Crossref] [PubMed]
- Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 2003;12:320-7. [Crossref] [PubMed]
- Fontaine C, Renard V, Van den Bulk H, et al. Weekly carboplatin plus neoadjuvant anthracycline-taxane-based regimen in early triple-negative breast cancer: a prospective phase II trial by the Breast Cancer Task Force of the Belgian Society of Medical Oncology (BSMO). Breast Cancer Res Treat 2019;176:607-15. [Crossref] [PubMed]
- Cámara RJA, Schwentner L, Friedl TWP, et al. Quality of life during and after adjuvant anthracycline-taxane-based chemotherapy with or without Gemcitabine in high-risk early breast cancer: results of the SUCCESS A trial. Breast Cancer Res Treat 2019;175:627-35. [Crossref] [PubMed]
- Untch M, Muscholl M, Tjulandin S, et al. First-line trastuzumab plus epirubicin and cyclophosphamide therapy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: cardiac safety and efficacy data from the Herceptin, Cyclophosphamide, and Epirubicin (HERCULES) trial. J Clin Oncol 2010;28:1473-80. [Crossref] [PubMed]
- Jamieson D, Lee J, Cresti N, et al. Pharmacogenetics of adjuvant breast cancer treatment with cyclophosphamide, epirubicin and 5-fluorouracil. Cancer Chemother Pharmacol 2014;74:667-74. [Crossref] [PubMed]
- Matikas A, Foukakis T, Moebus V, et al. Dose tailoring of adjuvant chemotherapy for breast cancer based on hematologic toxicities: further results from the prospective PANTHER study with focus on obese patients. Ann Oncol 2019;30:109-14. [Crossref] [PubMed]
- Han HS, Reis IM, Zhao W, et al. Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur J Cancer 2011;47:2537-45. [Crossref] [PubMed]
- Sandström M, Lindman H, Nygren P, et al. Population analysis of the pharmacokinetics and the haematological toxicity of the fluorouracil-epirubicin-cyclophosphamide regimen in breast cancer patients. Cancer Chemother Pharmacol 2006;58:143-56. [Crossref] [PubMed]
- Ackland SP, Gebski V, Zdenkowski N, et al. Dose intensity in anthracycline-based chemotherapy for metastatic breast cancer: mature results of the randomised clinical trial ANZ 9311. Breast Cancer Res Treat 2019;176:357-65. [Crossref] [PubMed]
- Coombes RC, Kilburn LS, Tubiana-Mathieu N, et al. Epirubicin dose and sequential hormonal therapy-Mature results of the HMFEC randomised phase III trial in premenopausal patients with node positive early breast cancer. Eur J Cancer 2016;60:146-53. [Crossref] [PubMed]
- Therasse P, Mauriac L, Welnicka-Jaskiewicz M, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. J Clin Oncol 2003;21:843-50. [Crossref] [PubMed]
- French Adjuvant Study Group. Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol 2001;19:602-11. [Crossref] [PubMed]
- Petit T, Borel C, Ghnassia JP, et al. Chemotherapy response of breast cancer depends on HER-2 status and anthracycline dose intensity in the neoadjuvant setting. Clin Cancer Res 2001;7:1577-81. [PubMed]
- von Minckwitz G, Raab G, Caputo A, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 2005;23:2676-85. [Crossref] [PubMed]
- Vriens BE, Aarts MJ, de Vries B, et al. Doxorubicin/cyclophosphamide with concurrent versus sequential docetaxel as neoadjuvant treatment in patients with breast cancer. Eur J Cancer 2013;49:3102-10. [Crossref] [PubMed]
- von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747-56. [Crossref] [PubMed]
- Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015;33:13-21. [Crossref] [PubMed]
- Zhou Y, Ouyang T, Xie Y, et al. A single-center, randomized, parallel controlled study comparing the efficacy and safety aspects of three anthracycline-based regimens as neoadjuvant chemotherapy in primary breast cancer. Breast Cancer Res Treat 2016;157:527-34. [Crossref] [PubMed]
- Pizzuti L, Barba M, Giannarelli D, et al. Neoadjuvant Sequential Docetaxel Followed by High-Dose Epirubicin in Combination With Cyclophosphamide Administered Concurrently With Trastuzumab. The DECT Trial. J Cell Physiol 2016;231:2541-7. [Crossref] [PubMed]