“Parathyroidectomy in pregnancy”—a single centre experience with review of evidence and proposal for treatment algorithim
Introduction
Primary hyperparathyroidism (PHPT) is a common endocrine disorder, affecting approximately 0.15% of the population (1). There is a greater prevalence amongst women, and as many as 25% of women may present during their childbearing years (2). However, there is a relative lack of data regarding the incidence of PHPT in pregnant women, with less than 200 cases described in previous reports (3-5). Potential explanations for this may be the physiological changes of pregnancy such as hypoalbuminaemia and increased glomerular filtration rate which may blunt the calcium response to PHPT (4). In addition, many PHPT patients present either with nonspecific symptoms or no symptoms at all, which may make the clinical diagnosis difficult to distinguish from pregnancy associated symptoms.
Management of these patients also poses a difficult question. The largest review of PHPT in pregnancy was carried out by Norman et al. and reviewed 32 patients with a total of 77 pregnancies (6). This study demonstrated an astonishingly high degree of risk to mother and foetus, with as many as 67% of mothers and 80% of foetuses experiencing harm. These findings were echoed by a recent and comprehensive literature review carried out by Diaz-Soto et al. (7). Reported maternal complications include nephrolithiasis, pancreatitis, and muscle weakness. The risk of pancreatitis is notably higher in pregnant patients with a frequency of approximately 10% in comparison to the 1% risk faced by non-pregnant patients with PHPT (7). The risk to the foetus includes intrauterine growth retardation, permanent hypoparathyroidism, prematurity, and intrauterine foetal loss. Severe hypercalcaemia carries a particularly bleak prognosis, with perinatal loss in up to 25%, although even mild forms are associated with a 3- to 5- fold increase in the rate of pregnancy loss (7). The risk was such that, although most elective surgery is normally delayed until after delivery, the recommendation has been made that parathyroidectomy be performed during pregnancy for all PHPT patients.
Despite this call for early intervention, there is a relative paucity of data regarding the best evidence approach to the optimal approach to undertaking parathyroidectomy in pregnancy. Sporadic cases have been reported with a variety of strategies for workup and operative approaches (8-10); but there are no available guidelines for perioperative workup and procedures. The aim of this series was to examine our experience in five cases of parathyroidectomy performed during pregnancy. To date, this is the largest case series report of surgical management of PHPT in pregnancy. Using this experience and a review of relevant literature, we then propose a step wise approach to management of the pregnant patient with PHPT.
Methods
This is a case series comprising five patients who underwent parathyroidectomy during pregnancy at our tertiary referral centre at St Thomas’ Hospital, London, England. Data collected included patient demographics and presenting features, pre- and post-operative biochemical markers, imaging, intraoperative findings, and postoperative maternal and foetal course.
Results
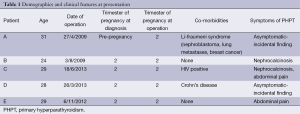
The demographic characteristics of the five patients and their presenting characteristics are detailed in Table 1.
Full table
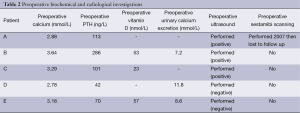
The results of preoperative biochemical and radiological investigations, where available, are presented in Table 2.
Full table
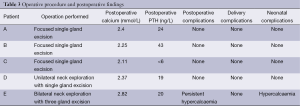
The operative procedure, postoperative findings, and complications related to operation, delivery, or neonate are presented in Table 3.
Full table
Final pathology in patients A-D revealed parathyroid adenoma in each; in patient E the parathyroid glands were hyperplastic but otherwise normal. Subsequent testing of patient E and her offspring revealed a mutation of the calcium sensing receptor (CaSR) consistent with familial hypocalciuric hypercalcaemia (FHH).
Conclusions
This case series illustrates both the successes and pitfalls which can be encountered when undertaking parathyroidectomy.
Diagnosis
As many as 80% of patients with PHPT may have no symptoms at all, and many cases may be picked up as an incidental finding of unexplained hypercalcaemia (11). Diagnosis of PHPT is classically based upon laboratory findings of elevated serum calcium with an inappropriately elevated PTH. Dias-Soto et al. (7) state that PHPT in pregnancy is probably underdiagnosed due to the masking effect of the maternal physiological adaptations lowering the observed serum calcium levels. Although standard antenatal screening in the UK does not include markers of PHPT, we would echo the call by Diaz-Soto et al. for measurement of calcium and PTH in pregnant patients with “classic” symptoms such as pancreatitis, fractures, or hyperemesis gravidarum; or indeed any symptoms of pregnancy which are prolonged and unexplained.
Differential diagnosis of hypercalcaemia includes PHPT and the rare autosomal dominant condition FHH. Urinary calcium excretion and the ratio of calcium:creatinine (Ca:Cr) clearance can be used to clarify diagnosis: PHPT demonstrates a high or high-normal calcium excretion with a Ca:Cr ratio of greater than 0.02; while FHH demonstrates a low urinary calcium excretion and Ca:Cr ratio of less than 0.01 (12). FHH is widely understood to demonstrate only a “mild” elevation in serum calcium and is often termed “Familial Benign Hypocalciuric Hypercalcaemia” due to the observed mild clinical syndrome (13). To our knowledge, this is the first report of evidence that FHH can cause extreme elevations of serum calcium, much greater than the “modest” elevations previously reported.
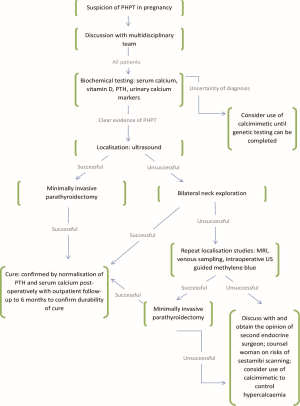
Clarity of diagnosis of PHPT and exclusion of FHH can be particularly difficult, as demonstrated by the case of Patient E. Patent E displayed characteristics of PHPT, with extreme elevation of serum calcium and inappropriate elevation of PTH. Bilateral neck exploration and excision of three glands was undertaken, but postoperatively showed no signs of improvement. With best medical management patient E completed her pregnancy with no significant complications, and delivered a healthy child who also demonstrated hypercalcaemia. After delivery, it was discovered that both patient E and her child had a congenital abnormality of the CaSR, consistent with FHH. Only extensive genetic testing, a lengthy and expensive process, can definitively confirm the diagnosis of FHH. Clearly, this is not practical to adopt as genetic testing as standard practice when there is clear time pressure to proceed to operation in these pregnant patients. Therefore, we would advocate an approach of building a likely diagnosis based on several pieces of key evidence. Firstly, and crucially based on our experience, we would no longer include an extreme elevation of serum calcium (above 3 mmol/L) alone as a key diagnostic factor for PHPT. Equally, an absolute reliance on hypocalciuria is not advisable for diagnosis of FHH as even confirmed patients may demonstrate normal urinary calcium excretion (14) and PHPT patients may demonstrate hypocalciuria if there is concomitant vitamin D deficiency. The presence of a single parathyroid mass on ultrasound may also falsely reassure one of the diagnoses of PHPT, as ultrasound can have a false positive rate of up to 21% (15). In summary: we would recommend a diagnostic approach which takes account of all three of these preoperative tests (serum calcium, urinary calcium markers of excretion and Ca:Cr ratio, and ultrasonography) and make a judgement of the balance of probability based upon them. Where there remains real and unacceptable uncertainty over diagnosis, the use of a calcimimetic such as cinacalcet has been demonstrated to have been used safely in pregnancy (16), and could be considered as a holding measure to control hypercalcaemia whilst genetic testing took place.
Localisation
The evolution of imaging techniques has seen a sea change in the approach to parathyroid surgery over the last 20 years, with accurate preoperative localisation studies permitting a “minimally invasive parathyroidectomy” (MIP) that targets only the identified likely culprit lesion. This has largely replaced the more traditional “bilateral neck exploration” as the gold standard operation for PHPT. The most accurate of the preoperative localisation studies is the 99m-technetium scan, which utilises the propensity for preferential uptake of the radioisotope by hyperfunctioning parathyroid tissue to correctly identify the site of adenoma in 88% of cases (17). Advances in CT scanning through rapid scanning and fine resolution have given rise to the development of “4D CT” imaging for identification of parathyroid adenomas, These images are developed through a four phase scanning technique with injection of an iodine rich contrast, allowing for examination of the uptake and washout of contrast by highly vascular tissue (i.e., parathyroid adenomas) within the neck and mediastinum. This has allowed 4D CT to be used for accurate localisation of the correct quadrant of disease, with reported accuracy of up to 87% (18).
Diagnostic tests using either high dose radiation or radioisotopes are almost universally avoided during pregnancy due to theoretical teratogenicity, and we would advocate the use of ultrasonography as the first line localising investigation in pregnant patients. Ultrasonography has accuracy of up to 79% in experienced hands (17), and for this reason we would suggest that all pregnant patients with PHPT have localisation scans at a specialist centre to maximise the likelihood of success. Specialist centres may also have the facility to offer ultrasound guided fine needle aspirate of lesions, with measurement of the PTH within the aspirate. This technique offers an excellent reported rate for confirmation of the parathyroid origin of tissue (up to 100% accuracy) (19) but is of course dependent of the successful localisation of a lesion to target for aspiration. If ultrasonography fails to identify a lesion, the surgeon is then faced with two options: to undertake BNE, or to request a 99m technetium scan. The woman should be counselled as to the risks and of each; namely the risk of recurrent laryngeal nerve palsy in BNE and the risk of administering radioactive material to the developing foetus with a 88% chance of successful localisation. There have been reports of the use of 99m-technetium in three parathyroidectomies and in several other procedures, with no reported immediate foetal or maternal complications. However, as is so often the case in pregnancy, large scale trials with long term follow up have not been conducted. The risk of radioisotope administration is therefore largely unquantified. By contrast, the risk of BNE to the recurrent laryngeal nerves has been extensively documented and is less than 1% (20). Therefore, in cases such as patient D where ultrasonography has failed to identify a culprit lesion, we would advocate proceeding to a planned bilateral neck exploration. In this case, the lesion was found at the first side of exploration and the procedure could be halted successfully (thus reducing yet further the risk of nerve injury).
MIP and BNE have similar success rates of 95% of patients achieving long term cure (21), but for the 5% failed first operation there then is the question of how to approach reoperative surgery. Again, the possibility of 99m-technetium scanning could be raised with the patient, although its usefulness is actually significantly reduced in the previously operated neck. Other options for localisation include noninvasive techniques such as MRI scanning (43-71% success) (17) or invasive PTH venous sampling (71-90%) (22). A recent report has suggested promising results in the use of intraoperative ultrasound guided methylene blue administration in the reoperative neck (23), and in future this may become a useful investigation in pregnant patients requiring reoperation.
Safety
The safety of operation is well illustrated by this series of patients, with no complications perioperatively or at delivery. There was one foetal complication of hypercalcaemia; however this was related to the child’s underlying pathological process of FHH rather than as a result of the operation. All our cases were operated on in the second trimester, and in several cases this required a rapid turnaround from time of first presentation to date of admission for surgery. There have been reports of parathyroidectomy being safely carried out in the third trimester (24), and this may be an option for women who present late into their pregnancy. However, there is no evidence to suggest superiority of outcomes in third trimester operating; indeed there may be exposure to unacceptable risk if operation is delayed. Therefore, it would be our recommendation that operation be carried out in the second trimester as soon as all preoperative investigations have been completed.
Operative approaches
As discussed above, the operative choice between MIP and BNE depends on the success of localisation techniques and also on surgeon experience. A review of operative approach in parathyroidectomy from 2010 stated that pregnancy is a positive predictive factor for BNE approach (25). However, there at least two case reports of focused parathyroidectomy being successfully carried out in pregnant patients with positive localisation studies (10,26).
An innovative compromise approach for the patient with negative localisation studies may be the “minimally invasive video assisted parathyroidectomy” (MIVAP) technique, which uses a small midline incision with retraction and endoscopic magnification to explore all four glands. This technique has been described by Bendinelli et al. (8) who describe a 15-mm incision through which all four glands could be explored, identified, and an adenoma removed. At present, MIVAP is rarely used in the adult patient due to the ability to perform MIP under direct vision. However, for the surgeon who has the skills and experience to perform MIVAP confidently can offer this approach as an excellent option for the patient with negative localisation studies.
The development of intra-operative of PTH monitoring (IOPTH) has provided endocrine surgeons with another useful adjunct during parathyroidectomy. The technique calls for serial measurements of IOPTH at induction of anaesthesia, or just prior to excision of the presumed culprit lesion followed by measurements at 5 and 10 minutes post-excision. The most widely used standard, the Miami criteria, uses a fall in IOPTH by 50% or greater at 10 minutes post-excision as evidence of successful operation (27). IOPTH monitoring can give reassurance to the surgeon that the target lesion has been correctly identified; and that exploration can be limited to that target site. IOPTH monitoring has been used successfully during pregnancy, as demonstrated by two case reports where excision of a single adenoma has been by fall in IOPTH in line with the Miami criteria. Limitations of IOPTH include the additional cost (although this has to be balanced against cost saved by limiting time spent on unnecessary exploration of the neck); and the lack of evidence of its efficacy in identifying multigland disease or double adenoma. However, where facilities for IOPTH monitoring are available, it may provide a useful adjunct to the surgeon performing parathyroidectomy in the pregnant patient and particularly in those with negative localisation studies.
Stepwise model
We have synthesised a summary of our experience and literature review into an evidence based stepwise model for planning approach to parathyroidectomy in the pregnant patient (Figure 1). In particular we would highlight the importance of a multidisciplinary team in managing these patients and their pregnancy. Given that the women in this series were all between the ages of 24-31, the number and severity of co-morbidities encountered is quite remarkable. To our knowledge there is no recognised association between Li-Fraumeni, HIV infection, or Crohn’s disease with PHPT. Rather, it is a reminder of the complexity of managing these patients which is best approached by a diverse multidisciplinary team. From time of first presentation, planning should be undertaken by a team experienced in parathyroid surgery and include the surgeon, anaesthetist, an experienced endocrine radiologist, obstetrician, neonatologist, and physicians involved in management of co-morbidities. We believe that by adopting a model based on best available evidence, coordinated by a multidisciplinary team, will prove beneficial in the management of parathyroidectomy in pregnancy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pallan S, Rahman MO, Khan AA. Hyperparathyroidism: diagnosis and management—a clinical review. BMJ 2012;344:e1013. [PubMed]
- Heath H 3rd, Hodgson SF, Kennedy MA. Primary hyperparathyroidism: incidence, morbidity, and potential economic impact in a community. N Engl J Med 1980;302:189-93. [PubMed]
- Schnatz PF, Curry SL. Primary hyperparathyroidism in pregnancy: evidence-based management. Obstet Gynecol Surv 2002;57:365-76. [PubMed]
- Amaya García M, Acosta Feria M, Soto Moreno A, et al. Primary hyperparathyroidism in pregnancy. Gynecol Endocrinol 2004;19:111-4. [PubMed]
- Jesudason WV, Murphy J, England RJ. Primary hyperparathyroidism in pregnancy. J Laryngol Otol 2004;118:891-2. [PubMed]
- Norman J, Politz D, Politz L. Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: a call for earlier intervention. Clin Endocrinol (Oxf) 2009;71:104-9. [PubMed]
- Diaz-Soto G, Linglart A, Sénat MV, et al. Primary hyperparathyroidism in pregnancy. Endocrine 2013;44:591-7. [PubMed]
- Bendinelli C, Nebauer S, Quach T, et al. Is minimally invasive parathyroid surgery an option for patients with gestational primary hyperparathyroidism? BMC Pregnancy Childbirth 2013;13:130. [PubMed]
- Petousis S, Kourtis A, Anastasilakis CD, et al. Successful surgical treatment of primary hyperparathyroidism during the third trimester of pregnancy. J Musculoskelet Neuronal Interact 2012;12:43-4. [PubMed]
- Malekar-Raikar S, Sinnott BP. Primary hyperparathyroidism in pregnancy-a rare cause of life-threatening hypercalcemia: case report and literature review. Case Rep Endocrinol 2011;2011:520516.
- Bilezikian JP, Silverberg SJ. Asymptomatic primary hyperparathyroidism. N Engl J Med 2004;350:1746-51. [PubMed]
- Endres DB. Investigation of hypercalcemia. Clin Biochem 2012;45:954-63. [PubMed]
- Christensen SE, Nissen PH, Vestergaard P, et al. Familial hypocalciuric hypercalcaemia: a review. Curr Opin Endocrinol Diabetes Obes 2011;18:359-70. [PubMed]
- Pasieka JL, Andersen MA, Hanley DA. Familial benign hypercalcaemia: hypercalciuria and hypocalciuria in affected members of a small kindred. Clin Endocrinol (Oxf) 1990;33:429-33. [PubMed]
- Jaskowiak N, Norton JA, Alexander HR, et al. A prospective trial evaluating a standard approach to reoperation for missed parathyroidadenoma. Ann Surg 1996;224:308-20; discussion 320-1. [PubMed]
- Edling KL, Korenman SG, Janzen C, et al. A pregnant dilemma: primary hyperparathyroidism due to parathyromatosis in pregnancy. Endocr Pract 2014;20:e14-7. [PubMed]
- Johnson NA, Tublin ME, Ogilvie JB. Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR Am J Roentgenol 2007;188:1706-15. [PubMed]
- Hunter GJ, Schellingerhout D, Vu TH, et al. Accuracy of four-dimensional CT for the localization of abnormal parathyroid glands in patients with primary hyperparathyroidism. Radiology 2012;264:789-95. [PubMed]
- Erbil Y, Barbaros U, Salmaslioglu A, et al. Value of parathyroid hormone assay for preoperative sonographically guided parathyroid aspirates for minimally invasive parathyroidectomy. J Clin Ultrasound 2006;34:425-9. [PubMed]
- Harris SC. Thyroid and parathyroid surgical complications. Am J Surg 1992;163:476-8. [PubMed]
- Sackett WR, Barraclough B, Reeve TS, et al. Worldwide trends in the surgical treatment of primary hyperparathyroidism in the era of minimally invasive parathyroidectomy. Arch Surg 2002;137:1055-9. [PubMed]
- Ito F, Sippel R, Lederman J, et al. The utility of intraoperative bilateral internal jugular venous sampling with rapid parathyroid hormone testing. Ann Surg 2007;245:959-63. [PubMed]
- Candell L, Campbell MJ, Shen WT, et al. Ultrasound-guided methylene blue dye injection for parathyroid localization in the reoperative neck. World J Surg 2014;38:88-91. [PubMed]
- Schnatz PF. Surgical treatment of primary hyperparathyroidism during the third trimester. Obstet Gynecol 2002;99:961-3. [PubMed]
- Norman J, Politz D. Prospective study in 3,000 consecutive parathyroid operations demonstrates 18 objective factors that influence the decision for unilateral versus bilateral surgical approach. J Am Coll Surg 2010;211:244-9. [PubMed]
- Pothiwala P, Levine SN. Parathyroid surgery in pregnancy: review of the literature and localization by aspiration for parathyroid hormone levels. J Perinatol 2009;29:779-84. [PubMed]
- Carneiro DM, Solorzano CC, Nader MC, et al. Comparison of intraoperative iPTH assay (QPTH) criteria in guiding parathyroidectomy: which criterion is the most accurate? Surgery 2003;134:973-9; discussion 979-81. [PubMed]