
Surgical approach to patients with pheochromocytoma
Introduction
Pheochromocytomas and paragangliomas (PPGLs) are rare neuroendocrine tumors comprised of chromaffin tissue that may secrete excess catecholamines including epinephrine, norepinephrine, dopamine and/or their metabolites including metanephrine, normetanephrine, and 3-methoxytyramine, respectively (1,2). Pheochromocytomas arise from the adrenal gland, whereas paragangliomas arise from the extra-adrenal chromaffin cells of the sympathetic and parasympathetic ganglia located in the neck, chest, abdomen, or pelvis. Nearly 80–85% of these chromaffin-comprised neuroendocrine tumors are pheochromocytomas, whereas 15–20% are extra-adrenal paragangliomas (3).
The annual incidence of pheochromocytomas is estimated to be between 500 to 1,600 cases per year (4) with an equal sex distribution and a peak in the fourth and fifth decades of life (5). The prevalence of pheochromocytomas in patients with hypertension is 0.1–0.6% (6,7). The classical presentation of pheochromocytomas consists of episodic hypertension, headaches, diaphoresis, and flushing in about 40% of patients (3). Up to 40% of patients with PPGLs have a genetic predisposition either from a familial predisposition or de novo mutation (8,9). Patients with PPGLs undergo biochemical testing of catecholamines and their metabolites to establish the diagnosis as well as anatomical and functional imaging as needed. After the biochemical diagnosis has been established, germline genetic testing completed, and imaging performed, the patient is offered surgery through either minimally invasive or open surgical approaches (1). The surgical approach and extent of adrenalectomy is personalized based on multiple factors including germline genetic test results, the size of the tumor, body mass index, surgeon experience, and the likelihood of malignancy.
The aim of this review is to describe the initial biochemical, genetic, and imaging work-up, preoperative optimization of the patient, potential surgical approaches to PPGLs, surgical techniques, postoperative management, and postoperative outcomes of patients with PPGLs.
Biochemical diagnosis
The biochemical diagnosis of PPGLs utilizes assays that measure catecholamines and their metabolites. Metanephrines, normetanephrines, and 3-methoxytyramine, which are metabolites of catecholamines, are consistently secreted in the serum, and this consistent secretion results in a higher sensitivity and specificity when diagnosing PPGLs compared to catecholamines. In a systematic review examining the accuracy of plasma free metanephrines and 24-hour urinary fractioned metanephrines, the most accurate conditions and biomarker for testing was a supine measurement of plasma free metanephrines, which yielded a sensitivity of 94%, specificity of 93%, and area of under the curve of 0.942 (10). Per the 2014 Endocrine Society Practice Guidelines, the initial workup may include either plasma free or 24-hour urinary fractionated metanephrines, which has comparable sensitivities and specificities to plasma free metanephrines (1). Although not widely available, if the clinician is concerned of malignant or metastatic disease, consideration should be given to measuring plasma 3-methoxytyramine levels. The measurement of 3-methoxytyramine levels were found to be 4.7-fold higher in patients with metastatic PPGLs (n=105) compared to patients without metastases (n=365). A tumor diameter greater than 5 cm (AUC =0.771, P<0.0001) and a plasma 3-methoxytyramine level greater than 0.2 nmol/L (AUC =0.739, P<0.0001) increases the likelihood of metastatic spread (2). An elevated 3-methoxytyramine level in combination with a large tumor may indicate the need for functional imaging to assess for metastatic disease and/or indicate an open surgical approach with lymphadenectomy for a potentially malignant pheochromocytoma or paraganglioma.
Genetic testing
The search for susceptibility genes has resulted in the discovery that up to 40% of patients with PPGLs are genetically inherited (8,9). Over the past couple of decades, clinical and translational research has identified over 20 germline and somatic mutations related to PPGLs including EPAS1, SDHA, SDHB, SDHC, SDHD, SDHAF2, VHL, EGLN1/2, FH, MDH2, IDH, RET, NF1, H-RAS, K-RAS, TMEM127, MAX, ATRX, CSDE1, MAML3, DNMT3A, SLC25A11, GOT2, IDH3B, KMT2D (9,11-15). In addition genotype-phenotype correlations have been described including a higher rate of metastatic disease for patients with SDHB mutations, a higher rate of head and neck paragangliomas and multiple paragangliomas for patients with SDHx mutations, and a higher rate of bilateral pheochromocytomas for patients with VHL, RET, MAX, and TMEM127 mutations (16). Given that up to 40% of patients with PPGLs have a germline genetic mutation, any patient diagnosed with a PPGL regardless of age or familial history should undergo genetic counseling and testing (1,17). Next Generation Sequencing (NGS) is the current gold-standard for genetic testing due to higher efficiency and cost-effectiveness than previous sequencing techniques. A 2017 consensus statement regarding genetic testing of PPGLs reported the common predisposing genes that should be tested as well as recommendations on the standardization of reporting results (18). Since the results have potential health and life insurance implications, patients should meet with a genetics counselor prior to testing. These genetic results can influence the extent of adrenalectomy as well as the surgical approach (see section Surgical Approaches).
Imaging
After biochemical confirmation of the diagnosis, anatomical imaging with either computed tomography (CT) and/or magnetic resonance imaging (MRI) is compulsory for surgical planning (Figure 1). Functional imaging utilizing a glucose radiotracer or a somatostatin analogue as an adjunct can be utilized when the clinician is concerned for metastatic disease or multisite disease.
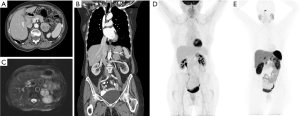
The typical appearance of a pheochromocytoma on CT is an unenhanced density greater than 10 Hounsfield units (HU), hyperintensity with contrast, and a delayed washout. Tumors may also have cystic changes, central necrosis, and internal calcifications. MRI can also be utilized, and shows T2 enhancement with contrast, which is the so-called light bulb sign. Tumors may also appear heterogenous due to central necrosis, cystic changes, or hemorrhage (19). 123I-Metaiodobenzylguanidine (123I-MIBG) was once a commonly used functional diagnostic imaging test with a sensitivity and specificity of 88% and 84%, respectively (20), but has been supplanted by newer radiotracers with higher sensitivity and specificity.
Newer functional imaging tracers have been compared in a head to head fashion for the detection of primary tumors as well as metastatic disease. To assess for localization of primary PPGLs and patients with PPGLs and metastatic disease, a study from the National Institutes of Health (NIH) compared the sensitivities and lesion counts of 18F-3, 4-dihyoxyphenylalanine (18F-DOPA), 18F-dopamine (18F-FDA), 18F-fluorodeoxyglucose (18F-FDG), and 123I-MIBG to CT/MRI scans. Twenty-eight patients had metastatic disease, and were found to have a total score of 334 lesions from all anatomic and functional imaging studies by regional analysis. The total score was highest for 18F-FDA PET/CT at 246, 211 for CT and/or MRI, 174 for 18F-DOPA PET/CT, and 209 for 18F-FDG PET/CT. The authors conclude that 18F-FDA PET/CT is the preferred technique for the localization of primary PPGLs and to detect metastatic disease (21).
Although 18F-FDA PET/CT was found to be superior amongst the functional imaging modalities, the radiotracer 18F-FDA is not commonly available outside of the NIH. A large prospective study of 216 patients with suspected PPGLs at the NIH comparing CT or MRI, 18F-FDG PET/CT and 123I-MIBG SPECT/CT showed CT/MRI to have the highest sensitivity in detecting non-metastatic tumors (95.7%) compared to 18F-FDG (76.8%) or 123I-MIBG (75.0%). The specificities were similar between the imaging modalities (~90%). However, 18F-FDG PET/CT had the highest sensitivity in detecting metastatic disease (82.5%) compared to CT/MRI (74.4%) or 123I-MIBG (50.0%). In the detection of bone metastases, 18F-FDG PET/CT (93.7%) was also superior to CT/MRI (76.7%) (22). 18F-FDG PET/CT scan should be utilized by the surgeon when concerned about patients with metastatic disease from PPGLs.
PPGLs may also be detected by 68Ga-DOTA(0)-Tyr(3)-octreotate (68Ga-DOTATATE) PET/CT scan (Figure 1). Like 18F-FDG PET/CT scan, the value of 68Ga-DOTATATE PET/CT scan is for assessing patients with PPGLs and metastatic disease. 68Ga-DOTATATE PET/CT scan has been shown to have a higher rate of lesion based detection rate of 97.6% compared to 18F-FDG PET/CT (49.2%), 18F-FDOPA PET/CT (74.8%), 18F-FDA PET/CT (77.7%) and CT/MRI (81.6%) (23). Given these impressive results, 68Ga-DOTATATE PET/CT scan may become the primary functional imaging modality when there is concern for metastatic disease and it has become more widely available in the United States.
Preoperative considerations
Surgical resection of PPGLs may result in labile blood pressure (BP), arrhythmias, and tachycardia in the perioperative period. Intraoperatively, patients undergoing surgical resection have been reported to have arrhythmias, sustained hypertension and/or hypotension, postoperative myocardial infarction, stroke, pulmonary edema, and prolonged intubation (24-27). Large tumor size, elevated preoperative levels of catecholamines, longer duration of surgery, preexisting catecholamine induced heart failure, and a technically difficult surgical resection have been associated with perioperative morbidity (25,26). Therefore, a multidisciplinary team with experienced surgeons, anesthesiologists, and endocrinologists is paramount to a safe outcome.
In the preoperative period, the goal is to optimize the BP and normalize the intravascular volume. There are no universally accepted algorithms, and clinical practice is institution based. However, experienced surgeons and endocrinologists agree that adequate preoperative optimization includes aiming for a seated BP of 120–130/80 mmHg, a standing systolic blood pressure (SBP) ≥90 mmHg, and a heart rate between 60 and 70 beats/minute (bpm) seated and 70 to 80 bpm standing. Patients are encouraged to be well hydrated with a high sodium diet prior to surgery (26). The timing and duration of preoperative optimization is unknown, but preoperative antihypertensive treatment usually lasts for at least 10 to 14 days (Table 1) (3).

Full table
Selective or non-selective alpha blockade is always initiated first in preoperative preparation of patients with PPGLs. Retrospective studies have addressed the benefits and drawbacks of selective and non-selective alpha blockade without a clear definitive superiority for one strategy. Phenoxybenzamine, a non-selective irreversible blocker of alpha receptors, has been reported to have slightly better perioperative hemodynamics, but with an increased rates of postoperative hypotension and higher rate of side effects compared to selective alpha blockers such as doxazosin or prazosin (28). Beta blockers are added as needed for tachycardia after at least three to four days of alpha blockade administration, because hypertension can worsen due to unopposed alpha-adrenergic stimulation (3). Calcium channel blockers can be used as an adjunct or as an alternative. A large retrospective analysis comparing 110 patients receiving calcium channel blockers compared to 41 patients receiving alpha blockers and 4 patients with no medications showed that intraoperative hemodynamic instability was independent of preoperative medical management (29). Metyrosine, a catecholamine synthesis inhibitor, is typically utilized for patients who cannot tolerate alpha blockers or have alpha blocker, beta blocker, and/or calcium channel blocker refractory hypertension (30).
Of note, there has been a large retrospective study showing that one may possibly forgo alpha blockade prior to surgery. In this observational study, 303 consecutive surgeries for pheochromocytomas were shown to have no difference in major cardiovascular complications between patients with and without alpha blockade. The maximal intraoperative SBP was, however, higher in patients without alpha blockade after propensity score matching. This study provides potential evidence that alpha blockade may be withheld in select centers with experienced surgeons and anesthesiologists (31). An important limitation to this study, however, is that 38.6% of patients without alpha blockade were on other undefined hypertensive medications, which could potentially include calcium channel blockers, and therefore confound the interpretation of the results. Despite this study, the most common recommendation continues to be initial alpha blocker with the possible addition of a beta blocker for tachycardia.
Anesthesia and intraoperative management
The communication between the anesthesiologist and the surgeon is paramount to a safe outcome. The appropriate intravenous access and an arterial catheter for continuous blood pressure monitoring should be placed preoperatively. The anesthesia team should be ideally experienced and prepared to control hemodynamic fluctuations with intravenous vasoactive medications.
Surgical approaches
The surgical approaches for patients with pheochromocytoma include open and minimally invasive techniques (Table 2). Minimally invasive techniques can be approached either laparoscopically or robotically. Important principles of both minimally invasive and open surgical resection include minimal manipulation of the tumor to prevent catecholamine release with resultant hemodynamic instability as well as tumor rupture. In addition, to reduce the likelihood of large amounts of catecholamines being released, early ligation of the adrenal vein is advocated (1,32,33).

Full table
Minimally invasive (laparoscopic or robotic) adrenalectomy is the preferred approach for patients with pheochromocytomas. This surgical approach may be performed either through the transabdominal approach (TA) or the posterior retroperitoneoscopic approach (PRA) (34-37). Ultimately, the surgical approach will be dependent on surgeon preference and familiarity with the techniques. However, factors that may influence surgical approach include body mass index, tumor size, tumor characteristics and location, and history of prior abdominal or retroperitoneal procedures (35,38-40).
The minimally invasive TA approach was first reported by Dr. Gagner and is the most familiar approach to most surgeons (41,42). The advantages include familiar anatomy and ease of conversion to open if necessary (43). The advantages of the PRA approach include direct access to the adrenal gland and decreased amount of dissection and mobilization of visceral organs to expose the adrenal glands (34,35). A comparison between the TA and PRA approach for patients with pheochromocytomas undergoing adrenalectomy showed that the PRA approach has shorter operative times (99.9 vs. 144.8 minutes, P<0.001), lower estimated blood loss (8.4 vs. 123.8 mL, P=0.02), and decreased postoperative length-of-stay (1.9 vs. 3.1 nights, P<0.01) in patients with similar tumor sizes and demographics (35). In addition, bilateral adrenalectomy without reposition is a benefit of the PRA approach. However, contraindications to the PRA approach include tumors larger than 7 to 8 cm due to the small working space and a high body mass index with increased retroperitoneal fat (34).
The TA and PRA approaches can be utilized for either laparoscopically or robotically. Retrospective studies have shown that robotic and laparoscopic resection of pheochromocytomas are equivalent in terms of operative time, estimated blood loss, conversion from minimally invasive to open, intraoperative hemodynamic instability, morbidity, and mortality (44). Advantages of robotic adrenalectomy include 3-dimensional view, improved wrist articulation, and a stable camera port. The disadvantages relative to laparoscopic adrenalectomy include increased cost, a learning curve, and the lack of haptic feedback (44,45).
The open approach is preferred when there is a clinical suspicion or concern for an invasive malignant pheochromocytoma. In addition, larger tumors are at a higher risk for tumor rupture, which may lead to pheochromocytomatosis. In case reports and small case series of tumor rupture leading to pheochromocytomatosis, the tumors are typically larger than 5.5 cm, but a tumor as small as 2.5 cm has been reported (46-48). An open approach may be preferred in patients with SDHB mutations, since patients with SDHB mutations have a higher rate of metastatic disease (49,50). A retrospective analysis of 108 patients with pheochromocytomas and paragangliomas who underwent preoperative genetic testing found that an open approach was associated with tumor size (P=0.009) and the presence of germline mutation (P=0.042). Given that patients with SDHB germline mutations have a higher rate of metastases and multisite disease, the authors found an association between SDHB mutation and open surgical approach (51). Since the rate of malignant disease in patients with SDHB has been reported to be as high as 34% (49), consideration for lymphadenectomy should be undertaken at the time of surgical resection. For patients with widely metastatic disease, there may be a role for cytoreductive surgery. Patients with malignant PPGLs with disease confined to the abdomen are more likely to achieve and maintain a biochemical response than those with extra-abdominal disease and may benefit from cytoreductive surgery (52).
A minimally invasive or open surgical approach can be utilized in cortical sparing (partial) adrenalectomies for patients with pheochromocytomas related to syndromes (Table 2). Patients with syndromic diseases such as multiple endocrine neoplasia 2 (MEN2) or von Hippel-Lindau disease (VHL) are offered cortical-sparing adrenalectomy to maximize adrenal function, avoid chronic steroid hormone replacement, and reduce the risk of Addisonian crisis (53). This approach has resulted from 78% to 89% of patients with syndromic diseases and cortically-spared adrenal glands to be steroid independent at long-term follow-up (51,53-55).
Minimally invasive techniques
Transabdominal approach
The patient is placed in a lateral decubitus position at a 90-degree angle to ensure gravity allows full retraction of the spleen on the left and partial retraction of the liver on the right. Three subcostal ports are placed on the left. Three subcostal ports and one epigastric port parallel to the inferior border of the liver are placed on the right. For laparoscopic left adrenalectomy, the spleen is mobilized until the gastric fundus is visualized, which allows the spleen to retract medially. The plane between the spleen and the tail of the pancreas, and the left adrenal gland is developed. For laparoscopic right adrenalectomy, the right triangular ligament is divided and the right lobe of the liver retracted medially through the epigastric port. The plane between the inferior vena cava, and the right adrenal gland is developed. For either side, the adrenal vein is identified, meticulously dissected and isolated, and divided between clips. Soft tissue attachments are then divided with the Harmonic Scalpel (Ethicon Endo-Surgery, Cincinnati, OH). The specimen is placed in an Endo Catch device and removed.
Posterior retroperitoneoscopic approach
The patient is placed in a jackknife position on a Cloward Surgical Saddle (Surgical Equipment International, Honolulu, HI). A 1.5 cm transverse incision is placed just below the tip of the 12th rib to enter the retroperitoneum space. Digital palpation is used to develop the space. A medial trocar is placed along the lateral border of the paraspinous muscle using the surgeon’s index finger as a guide. A lateral trocar is placed below the 11th rib in a similar fashion. A 12 mm blunt balloon trocar (Ethicon Endosurgery) is inserted through the initial incision. Pneumoperitoneum is established with CO2 insufflation, which is maintained at 20–24 mmHg. The working space is then developed by dissecting the retroperitoneal areolar tissue and Gerota’s fascia to identify the superior border of the kidney. The lower aspect of either the left or right gland is retracted caudally. The adrenal gland is dissected laterally and inferiorly. The superior and medial aspects are dissected last with the identification of the adrenal vein. The adrenal vein is identified, dissected, and divided between clips. The superior attachments are then released, and the specimen is placed into an Endo Catch device and removed.
Open techniques
There are multiple incisions that can be utilized for exposure including subcostal, midline, and the Makuuchi incision. The author’s preference is to utilize subcostal incision two to three fingerbreadths below the costal margin. This incision provides excellent exposure of the liver, as well as the adrenal bed. In addition, if a lymphadenectomy is planned, a subcostal incision will allow access to the aortocaval space, and the perihilar lymph nodes. As with the laparoscopic technique, the triangular ligament is mobilized on the right and the spleen is mobilized on the left to access the retroperitoneum. Important landmarks to identify on the right include the inferior vena cava and on the left the avascular plane between the spleen and tail of the pancreas, and the left adrenal gland. For either side, the adrenal vein is identified and clipped. Any remaining soft tissue attachments are divided and the specimen is removed.
Cortical sparing techniques
Once the adrenal gland has been exposed by either mobilization of the triangular ligament on the right side or the spleen on the left side, the tumor may be visible as a mass within the retroperitoneum. At this point, it is prudent to perform an ultrasound to assess the relationship of the tumor and the adrenal vein, and to identify whether there are multiple nodules in those with a genetic predisposition. Utilizing ultrasound to identify key anatomy, the surgeon should attempt to preserve the adrenal vein to allow for adequate function of the adrenal remnant. If there is a solitary nodule, the Harmonic Scalpel is used to enucleate the nodule. Since the adrenal gland is highly vascular, activation of the harmonic with the jaws open while slowly clamping down on the adrenal tissue provides ideal hemostasis. Of note, the nodule or the adrenal gland should not be grasped at any point. Grasping either the nodule or the adrenal gland may result in fracturing of the nodule and/or gland, which could lead to pheochromocytomatosis. Grasping a portion of adipose tissue attached to the adrenal gland or using gentle retraction will give the appropriate exposure for enucleation. Once the nodule has been enucleated, the remnant adrenal gland should be examined for hemostasis. If there are multiple nodules amenable to enucleations, the surgeon should proceed as previous. However, if there are multiple nodules and the remnant gland will be an estimated less than 30% of the whole, then the surgeon should consider a total adrenalectomy. The remnant gland will likely be nonfunctional, and the patient may benefit from a total adrenalectomy to avoid needing reoperative surgery for a recurrence. Once the tumor(s) are enucleated, the specimen is placed in an Endo Catch device and removed.
Postoperative management
Postoperatively, most patients who have had an uneventful intraoperative course with minimal hemodynamic instability are admitted to the floors. Patients who have ongoing hemodynamic instability may require a monitored bed with telemetry or the intensive care unit. In the immediate postoperative period, patients with pheochromocytoma are at risk of hypotension and hypoglycemia (56). Transient episodes of hypotension are common and are attributed to residual preoperative alpha blockade, hypovolemia secondary to preoperative volume contract, and intraoperative blood loss. Treatment includes aggressive IV hydration and/or transient vasopressors (3).
A relatively rare risk postoperatively is hypoglycemia after surgical resection. Preoperatively, elevated catecholamine levels cause suppression of alpha and beta cell function, which results in increased insulin resistance. The risk of hypoglycemia is thought to be due to a reduction in alpha-2 receptor stimulation by the rapidly decreasing levels of circulating serum catecholamines after surgical resection (56). The rate of hypoglycemia after surgery has been reported from 4.2% to 15% (56-58). Independent predictors associated with postoperative hypoglycemia include elevated preoperative 24-hour urine epinephrine and longer operative time (56), and therefore blood glucose should be periodically checked postoperatively for the first 24 hours postoperatively.
Postoperative outcomes
The morbidity of untreated pheochromocytoma is difficult to ascertain. The best approximate data to the natural history of untreated pheochromocytoma can be ascertained from autopsy studies. When assessing the cause of death for patients with undiagnosed and untreated pheochromocytoma at autopsy, 71% of these patients died from cardiovascular causes such as myocardial infarction, hypertensive heart failure, strokes, or hemodynamic crises precipitated by or occurring during unrelated procedures (59). Patients with pheochromocytomas have a 14-fold increased risk of cardiovascular events such as myocardial infarction, stroke, or angina pectoris within 5 years prior to diagnosis compared to patients with essential hypertension (60). A retrospective study examining the outcomes of 64 patients with benign pheochromocytomas showed that 64% of patients had a decrease in blood pressure and one third of patients were normotensive (61). Although these outcomes are beneficial, the risk of mortality is higher for patients with pheochromocytoma compared to the general population. In a long-term study of 121 patients with pheochromocytomas after adrenalectomy, 42 patients with pheochromocytoma died compared to an estimated 23.6 expected deaths in the general Swedish population. In addition, with an average follow-up of 15 years, there were eight patients with recurrences due to local recurrence (n=2), distant recurrences (n=5), and both local and distant recurrences (n=1) (62).
Postoperative outcomes for cortical-sparing adrenalectomy
The extent of adrenalectomy can be dictated by genetic and familial predisposition. Patients with familial syndromes such as MEN 2A, MEN 2B, and VHL are ideal candidates for cortical sparing adrenalectomies. Cortical sparing adrenalectomies may prevent and forestall steroid dependency in these patients. However, the benefit of avoiding steroid dependency must be weighed against the risk of tumor recurrence.
Patients with MEN 2 who undergo cortical sparing adrenalectomy have a risk of recurrence as high as 51.8% at 10 years compared to no recurrences for patients who underwent bilateral total adrenalectomy. Although the risk of recurrence was zero at 10 years for these patients, 2 of the nine patients had an Addisonian crisis including one patient who died (63). A large retrospective observational study with 563 patients with MEN 2 and pheochromocytomas reported the results of 114 patients who underwent a cortical sparing adrenalectomy. When analyzed by operated gland, four out of 153 (3%) cortically-spared glands had an ipsilateral recurrence with a mean follow-up of 10 years compared to eleven out of 717 (2%) of total adrenalectomy patients had a recurrence within the ipsilateral adrenal bed with a mean follow-up of 14.2 years. The rate of steroid dependency of those patients who underwent either unilateral or bilateral cortical sparing adrenalectomy was 43% (64).
Cortical sparing adrenalectomy is equally encouraging for patients with VHL syndrome. At the NIH, 26 patients with VHL were seen over 8 years. These patients underwent 36 partial adrenalectomies with a median follow-up of 9.2 years (5–46 years). Eleven percent of patients developed recurrence within the ipsilateral adrenal gland remnant and 11% of patients developed a recurrent pheochromocytoma within the contralateral adrenal gland requiring a partial adrenalectomy. In this series of patients, only 11% of patients became steroid dependent and no patients developed metastatic disease (55).
Conclusions
The surgical approach to pheochromocytomas is based on assessing imaging, identifying germline genetic mutations, and utilizing minimally invasive techniques when feasible and indicated. Appropriate imaging enables assessment of malignancy and extent of disease. Identification of the germline genetic mutations determines whether cortical sparing adrenalectomy is indicated in syndromic patients or if an open approach and/or lymphadenectomy may be beneficial in SDHB germline mutation patients. Minimally invasive techniques either approached transabdominally or retroperitoneally is the standard of care. Open surgical approaches are reserved for large tumors at risk for rupture and potentially malignant tumors. Outcomes after surgical resection are beneficial and may reduce the cardiovascular morbidity of this patient population.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915-42. [Crossref] [PubMed]
- Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer 2012;48:1739-49. [Crossref] [PubMed]
- Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet 2005;366:665-75. [Crossref] [PubMed]
- Chen H, Sippel RS, O'Dorisio MS, et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas 2010;39:775-83. [Crossref] [PubMed]
- Guerrero MA, Schreinemakers JM, Vriens MR, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg 2009;209:727-32. [Crossref] [PubMed]
- Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens 1994;12:609-15. [Crossref] [PubMed]
- Omura M, Saito J, Yamaguchi K, et al. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res 2004;27:193-202. [Crossref] [PubMed]
- Babic B, Patel D, Aufforth R, et al. Pediatric patients with pheochromocytoma and paraganglioma should have routine preoperative genetic testing for common susceptibility genes in addition to imaging to detect extra-adrenal and metastatic tumors. Surgery 2017;161:220-7. [Crossref] [PubMed]
- Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol 2015;11:101-11. [Crossref] [PubMed]
- Därr R, Kuhn M, Bode C, et al. Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: a systematic review. Endocrine 2017;56:495-503. [Crossref] [PubMed]
- Remacha L, Curras-Freixes M, Torres-Ruiz R, et al. Gain-of-function mutations in DNMT3A in patients with paraganglioma. Genet Med 2018;20:1644-51. [Crossref] [PubMed]
- Remacha L, Comino-Mendez I, Richter S, et al. Targeted Exome Sequencing of Krebs Cycle Genes Reveals Candidate Cancer-Predisposing Mutations in Pheochromocytomas and Paragangliomas. Clin Cancer Res 2017;23:6315-24. [Crossref] [PubMed]
- Buffet A, Morin A, Castro-Vega LJ, et al. Germline Mutations in the Mitochondrial 2-Oxoglutarate/Malate Carrier SLC25A11 Gene Confer a Predisposition to Metastatic Paragangliomas. Cancer Res 2018;78:1914-22. [Crossref] [PubMed]
- Juhlin CC, Stenman A, Haglund F, et al. Whole-exome sequencing defines the mutational landscape of pheochromocytoma and identifies KMT2D as a recurrently mutated gene. Genes Chromosomes Cancer 2015;54:542-54. [Crossref] [PubMed]
- Fishbein L, Leshchiner I, Walter V, et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017;31:181-93. [Crossref] [PubMed]
- Vicha A, Musil Z, Pacak K. Genetics of pheochromocytoma and paraganglioma syndromes: new advances and future treatment options. Curr Opin Endocrinol Diabetes Obes 2013;20:186-91. [Crossref] [PubMed]
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 2015;17:70-87. [Crossref] [PubMed]
- Group NGSiPS, Toledo RA, Burnichon N, et al. Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol 2017;13:233-47. [Crossref] [PubMed]
- Baez JC, Jagannathan JP, Krajewski K, et al. Pheochromocytoma and paraganglioma: imaging characteristics. Cancer Imaging 2012;12:153-62. [PubMed]
- Wiseman GA, Pacak K, O'Dorisio MS, et al. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. J Nucl Med 2009;50:1448-54. [Crossref] [PubMed]
- Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2009;94:4757-67. [Crossref] [PubMed]
- Timmers HJ, Chen CC, Carrasquillo JA, et al. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst 2012;104:700-8. [Crossref] [PubMed]
- Janssen I, Chen CC, Millo CM, et al. PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 2016;43:1784-91. [Crossref] [PubMed]
- Goldstein RE, O'Neill JA Jr, Holcomb GW 3rd, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg 1999;229:755-64; discussion 764-6. [Crossref] [PubMed]
- Kinney MA, Warner ME, vanHeerden JA, et al. Perianesthetic risks and outcomes of pheochromocytoma and paraganglioma resection. Anesth Analg 2000;91:1118-23. [PubMed]
- Kercher KW, Novitsky YW, Park A, et al. Laparoscopic curative resection of pheochromocytomas. Ann Surg 2005;241:919-26; discussion 926-8. [Crossref] [PubMed]
- Tauzin-Fin P, Hilbert G, Krol-Houdek M, et al. Mydriasis and acute pulmonary oedema complicating laparoscopic removal of phaechromocytoma. Anaesth Intensive Care 1999;27:646-9. [Crossref] [PubMed]
- van der Zee PA, de Boer A. Pheochromocytoma: a review on preoperative treatment with phenoxybenzamine or doxazosin. Neth J Med 2014;72:190-201. [PubMed]
- Brunaud L, Boutami M, Nguyen-Thi PL, et al. Both preoperative alpha and calcium channel blockade impact intraoperative hemodynamic stability similarly in the management of pheochromocytoma. Surgery 2014;156:1410-7; discussion 1417-8. [Crossref] [PubMed]
- Steinsapir J, Carr AA, Prisant LM, et al. Metyrosine and pheochromocytoma. Arch Intern Med 1997;157:901-6. [Crossref] [PubMed]
- Groeben H, Nottebaum BJ, Alesina PF, et al. Perioperative alpha-receptor blockade in phaeochromocytoma surgery: an observational case series. Br J Anaesth 2017;118:182-9. [Crossref] [PubMed]
- Kiernan CM, Du L, Chen X, et al. Predictors of hemodynamic instability during surgery for pheochromocytoma. Ann Surg Oncol 2014;21:3865-71. [Crossref] [PubMed]
- Livingstone M, Duttchen K, Thompson J, et al. Hemodynamic Stability During Pheochromocytoma Resection: Lessons Learned Over the Last Two Decades. Ann Surg Oncol 2015;22:4175-80. [Crossref] [PubMed]
- Walz MK, Alesina PF, Wenger FA, et al. Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients. Surgery 2006;140:943-8; discussion 948-50. [Crossref] [PubMed]
- Dickson PV, Alex GC, Grubbs EG, et al. Posterior retroperitoneoscopic adrenalectomy is a safe and effective alternative to transabdominal laparoscopic adrenalectomy for pheochromocytoma. Surgery 2011;150:452-8. [Crossref] [PubMed]
- Nigri G, Rosman AS, Petrucciani N, et al. Meta-analysis of trials comparing laparoscopic transperitoneal and retroperitoneal adrenalectomy. Surgery 2013;153:111-9. [Crossref] [PubMed]
- Li QY, Li F. Laparoscopic adrenalectomy in pheochromocytoma: retroperitoneal approach versus transperitoneal approach. J Endourol 2010;24:1441-5. [Crossref] [PubMed]
- Perrier ND, Kennamer DL, Bao R, et al. Posterior retroperitoneoscopic adrenalectomy: preferred technique for removal of benign tumors and isolated metastases. Ann Surg 2008;248:666-74. [PubMed]
- Agcaoglu O, Sahin DA, Siperstein A, et al. Selection algorithm for posterior versus lateral approach in laparoscopic adrenalectomy. Surgery 2012;151:731-5. [Crossref] [PubMed]
- Lee CR, Walz MK, Park S, et al. A comparative study of the transperitoneal and posterior retroperitoneal approaches for laparoscopic adrenalectomy for adrenal tumors. Ann Surg Oncol 2012;19:2629-34. [Crossref] [PubMed]
- Gagner M, Lacroix A, Prinz RA, et al. Early experience with laparoscopic approach for adrenalectomy. Surgery 1993;114:1120-4; discussion 1124-5. [PubMed]
- Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Berber E, Tellioglu G, Harvey A, et al. Comparison of laparoscopic transabdominal lateral versus posterior retroperitoneal adrenalectomy. Surgery 2009;146:621-5; discussion 625-6. [Crossref] [PubMed]
- Aliyev S, Karabulut K, Agcaoglu O, et al. Robotic versus laparoscopic adrenalectomy for pheochromocytoma. Ann Surg Oncol 2013;20:4190-4. [Crossref] [PubMed]
- Taskin HE, Berber E. Robotic adrenalectomy. J Surg Oncol 2012;106:622-5. [Crossref] [PubMed]
- Rafat C, Zinzindohoue F, Hernigou A, et al. Peritoneal implantation of pheochromocytoma following tumor capsule rupture during surgery. J Clin Endocrinol Metab 2014;99:E2681-5. [Crossref] [PubMed]
- Yu R, Sharaga D, Donner C, et al. Pheochromocytomatosis associated with a novel TMEM127 mutation. Endocrinol Diabetes Metab Case Rep 2017;2017. [Crossref] [PubMed]
- Li ML, Fitzgerald PA, Price DC, et al. Iatrogenic pheochromocytomatosis: a previously unreported result of laparoscopic adrenalectomy. Surgery 2001;130:1072-7. [Crossref] [PubMed]
- Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 2004;292:943-51. [Crossref] [PubMed]
- Gimenez-Roqueplo AP, Favier J, Rustin P, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res 2003;63:5615-21. [PubMed]
- Nockel P, El Lakis M, Gaitanidis A, et al. Preoperative genetic testing in pheochromocytomas and paragangliomas influences the surgical approach and the extent of adrenal surgery. Surgery 2018;163:191-6. [Crossref] [PubMed]
- Ellis RJ, Patel D, Prodanov T, et al. Response after surgical resection of metastatic pheochromocytoma and paraganglioma: can postoperative biochemical remission be predicted? J Am Coll Surg 2013;217:489-96. [Crossref] [PubMed]
- Lee JE, Curley SA, Gagel RF, et al. Cortical-sparing adrenalectomy for patients with bilateral pheochromocytoma. Surgery 1996;120:1064-70; discussion 1070-1. [Crossref] [PubMed]
- Grubbs EG, Rich TA, Ng C, et al. Long-term outcomes of surgical treatment for hereditary pheochromocytoma. J Am Coll Surg 2013;216:280-9. [Crossref] [PubMed]
- Benhammou JN, Boris RS, Pacak K, et al. Functional and oncologic outcomes of partial adrenalectomy for pheochromocytoma in patients with von Hippel-Lindau syndrome after at least 5 years of followup. J Urol 2010;184:1855-9. [Crossref] [PubMed]
- Chen Y, Hodin RA, Pandolfi C, et al. Hypoglycemia after resection of pheochromocytoma. Surgery 2014;156:1404-8; discussion 1408-9. [Crossref] [PubMed]
- Akiba M, Kodama T, Ito Y, et al. Hypoglycemia induced by excessive rebound secretion of insulin after removal of pheochromocytoma. World J Surg 1990;14:317-24. [Crossref] [PubMed]
- Plouin PF, Duclos JM, Soppelsa F, et al. Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: analysis of 165 operations at a single center. J Clin Endocrinol Metab 2001;86:1480-6. [PubMed]
- Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin Proc 1981;56:354-60. [PubMed]
- Stolk RF, Bakx C, Mulder J, et al. Is the excess cardiovascular morbidity in pheochromocytoma related to blood pressure or to catecholamines? J Clin Endocrinol Metab 2013;98:1100-6. [Crossref] [PubMed]
- Timmers HJ, Brouwers FM, Hermus AR, et al. Metastases but not cardiovascular mortality reduces life expectancy following surgical resection of apparently benign pheochromocytoma. Endocr Relat Cancer 2008;15:1127-33. [Crossref] [PubMed]
- Khorram-Manesh A, Ahlman H, Nilsson O, et al. Long-term outcome of a large series of patients surgically treated for pheochromocytoma. J Intern Med 2005;258:55-66. [Crossref] [PubMed]
- Asari R, Scheuba C, Kaczirek K, et al. Estimated risk of pheochromocytoma recurrence after adrenal-sparing surgery in patients with multiple endocrine neoplasia type 2A. Arch Surg 2006;141:1199-205; discussion 1205. [Crossref] [PubMed]
- Castinetti F, Qi XP, Walz MK, et al. Outcomes of adrenal-sparing surgery or total adrenalectomy in phaeochromocytoma associated with multiple endocrine neoplasia type 2: an international retrospective population-based study. Lancet Oncol 2014;15:648-55. [Crossref] [PubMed]


