Primary aldosteronism diagnostics: KCNJ5 mutations and hybrid steroid synthesis in aldosterone-producing adenomas
Introduction
Primary aldosteronism (PA) is the most common form of secondary hypertension, and it accounts for 5–8% of hypertension (1-6) and 11–20% of resistant hypertension (7-9). PA is characterized by inappropriate autonomous production of aldosterone via renin-independent mechanisms (10). Most PA patients exhibit a sporadic form, whereas 5–6% of the cases are caused by familial disease (11). Approximately 60–70% of PA cases can be attributed to bilateral hyperaldosteronism (BHA), with the remaining 30–40% being caused by unilateral aldosterone-producing adenomas (APA) (2,12). Uncommon forms of PA include unilateral adrenal hyperplasia and adrenal carcinoma (13).
Differentiation of the uni- and bilateral forms of PA is important for guiding therapy. Unilateral PA can benefit from adrenalectomy and BHA requires indefinite medical therapy, which typically incorporates a mineralocorticoid receptor antagonists (MRA) (14,15). Adrenal vein sampling (AVS) is the most reliable method for distinguishing between APA and BHA (10). However, there are several caveats to the AVS methodology and interpretation of hormonal data. Despite being highly predictive of outcome (10), this procedure is laborious, invasive, and expensive. Additionally, AVS is performed in a limited number of referral centers, and it is dependent on highly-skilled interventional radiologists with large annual AVS volume (16-18).
A simple blood test that identifies APA-derived serum steroid biomarkers would help distinguish patients who will benefit from adrenalectomy from those who should be treated with medical therapy. Such biomarkers could also conserve healthcare resources by sparing many patients expensive imaging and invasive studies. Finally, the utility of such biomarkers would increase the rate of PA screening, facilitate PA diagnosis and appropriate treatment, and thus reduce the burden of cardiovascular and renal complications which affect PA patents disproportionately more than those with essential hypertension.
Hybrid steroids as diagnostic markers for differentiation of APA from BHA
Over the past 25 years, the applicability of the 18-oxygenated derivatives of cortisol, 18-oxocortisol (18oxoF) and 18-hydroxycortisol (18OHF) for subtyping PA as uni- or bilateral, has been of interest (19-27). Several studies have shown higher levels of 18OHF and 18oxoF in PA patients compared to those with essential hypertension (23,28-30). High concentrations were also demonstrated in patients with familial hyperaldosteronism type 1 (FH type I) (31,32). FH type I accounts for <1% of cases of PA (11) and is caused by a chimeric gene that is composed of the promoter of 11β-hydroxylase (CYP11B1) fused with the coding region of aldosterone synthase (CYP11B2) (31). Like CYP11B1, this CYP11B1/CYP11B2 chimeric enzyme is present in the zona fasciculata (ZF) of the adrenal cortex and is regulated by ACTH. As a result, the CYP11B1/CYP11B2 chimera is able to use cortisol as a substrate to produce 18OHF, which is further metabolized to 18oxoF (19,23,30,32-34) (Figure 1). These metabolites of cortisol are designated as “hybrid” steroids owing to their molecular structure comprising features of steroid metabolism which typically occur in the zona glomerulosa (ZG) (18-hydroxylation and 18-oxidation) and the ZF (17-hydroxylation) (31) (Figure 1).
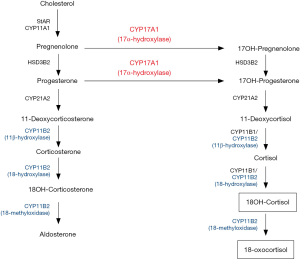
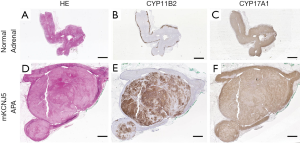
In the normal adrenal gland, expression of CYP11B2 is restricted to the ZG, while 17α-hydroxylase/17,20-lyase (CYP17A1) and CYP11B1 are expressed exclusively in the ZF and zona reticularis (Figure 2), thereby leading to low production of 18OHF and 18oxoF in normal subjects. Histologic studies have shown that some APA display a ZG-like phenotype, with small, compact cells, while other APA are composed of large, lipid-rich cells, similar to those seen in ZF (35-38). Although transcriptomic analysis has not detected any differences in CYP11B2 mRNA expression between the different APA subtypes (39), higher CYP11B2 protein expression has been observed in the APA with ZG-like cells in some studies (35,37). Specific to APA with ZF-like histology is a higher expression of steroidogenic enzymes required for cortisol biosynthesis, such as CYP17A1 and CYP11B1 (40-42). The co-expression of CYP17A1 and CYP11B2 in these APA facilitates the production of hybrid steroids (21,33,43) (Figures 1,2).
Ulick et al. performed the initial PA hybrid steroid studies in 1993 and demonstrated that urinary 18OHF and 18oxoF were elevated in patients with APA compared to those with BHA (21,22). Numerous immunoassay studies followed that indicated higher plasma and urinary 18OHF and 18oxoF levels in subjects with APA vs. those with BHA (19,23,29). More recently liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to quantify the hybrid steroids in APA and BHA, confirming some of the previous immunoassay analyses (20,25). Satoh et al. measured 18OHF and 18oxoF in the peripheral plasma of 234 Japanese PA patients by LC-MS/MS and found that these steroids could discriminate APA from BHA with considerable specificity and sensitivity (20). In contrast, a European study of 216 PA patients indicated that both 18oxoF and 18OHF displayed significant overlap between APA and BHA, thereby suggesting a limitation in the utility of these steroids as discriminators between the two PA subtypes (24). Nevertheless, this analysis presented a composite of 12 steroids that was able to correctly classify the PA subtype in 80% of the patients (24). A subsequent study from the same group revealed that APA with different underlying somatic mutations produce specific steroid fingerprints (44).
The hybrid steroids and KCNJ5 somatic mutation connection in APA
In 2016, Williams et al. identified specific steroid fingerprints in adrenal vein (AV) and peripheral vein (PV) plasma from patients with APA with various underlying mutations (44). Of the 79 PA patients with unilateral PA included, 34% had APA harboring KCNJ5 (encoding the G protein-coupled inward-rectifying potassium channel 4, GIRK4) mutations, 11% had ATPase (ATP1A1 (Na+/K+ ATPase α1-subunit), ATP2B3 (Ca2+ ATPase 3) mutations and 9% had CACNA1D (encoding the voltage-dependent L-type calcium channel subunit α-1D, Cav1.3) mutations. In the remaining 46% of APA no mutations were identified. Patients with KCNJ5-harboring APA had the highest concentrations of 18OHF and 18oxoF in both the AV and PV plasma (44). The elevated levels of the hybrid steroids produced by the KCNJ5-mutated APA could be explained by their predominantly ZF phenotype (elevated CYP17A1) along with CYP11B2 expression (35-38) (Figure 2). Conversely, APA with ATP1A1, ATP2B3, and CACNA1D mutations, were shown to be smaller in size and to be composed principally of ZG-like cells (35-38). Assembling a 7-steroid panel measured in PV plasma, including aldosterone, 18oxoF, 18OHF, 11-deoxycorticosterone (11-DOC), corticosterone, cortisol, and 21-deoxycortisol, William et al. were able to classify 92% of APA according to the underlying somatic mutation (44). In a subsequent study, the same group used steroid profiling to differentiate patients with micro-APA, macro-APA and BHA (45). Patients with macro-APAs, which frequently harbor KCNJ5 mutations, displayed higher concentrations of aldosterone and the hybrid steroids as compared with patients with micro-APA and BHA (45). These findings were in concordance with a recent analysis of APA tissue by comprehensive mass spectrometry imaging in relation to mutation status, immunohistochemical reports of steroidogenic enzymes and steroid profiles from 139 patients (46). Increased intratumoral intensities of 18OHF and 18oxoF were seen in KCNJ5-mutated APA. Additionally, two in vitro studies corroborated the finding that expression of a KCNJ5 mutation in the adrenocortical HAC15 cell line results in a significant increase in CYP11B2 gene transcription, and elevation in the production of aldosterone and the hybrid steroids (47,48).
The utility of hybrid steroids as promising discriminators between APA and BHA in Japanese patients with PA (20) could be attributed to the high prevalence of APA KCNJ5 mutations in this population (49,50). Tezuka et al. recently highlighted the potential of 18oxoF as biomarker for KCNJ5-harboring APA in Japanese patients in a study that measured its intratumoral and peripheral serum levels in patients with PA who underwent unilateral adrenalectomy (51). This study showed that APA harboring KCNJ5 mutations demonstrated enhanced synthesis of 18oxoF owing to elevated intratumoral cortisol production which could be used as substrate by CYP11B2. These tumors also had increased CYP11B1 and CYP11B2 double-positive hybrid cells compared with APA harboring the wild-type KCNJ5 gene (51). In addition to aberrant KCNJ5-related sporadic PA, the hybrid steroids have also been shown to be elevated in familial hyperaldosteronism type III (FH type III) (52,53) which was described by Geller et al. in 2008 as an early onset and severe form of primary aldosteronism (52) and was shown to be caused by germline mutations in KCNJ5 (53).
Somatic KCNJ5 mutations in APA—the mechanics
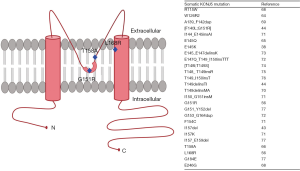
The product of KCNJ5, GIRK4 is a member of the G protein-activated inwardly rectifying K+ channel subfamily and is localized on the plasma membrane of tissues such as the heart, central and peripheral neurons along with various endocrine tissues (54,55). Tissue transcriptome analysis, however, suggests that the adrenal is by far the tissue with highest levels of the transcript encoding GIRK4. ‘Inward rectifiers’ are a class of K+ channels that conduct large currents in the inward direction at membrane voltages negative to the K+ equilibrium potential. The primary structure of this channel consists of 2 membrane spanning helices flanking one extracellular pore-forming region in between and cytoplasmic N- and C-termini that contribute to the pore structure (56,57). The pore-forming domain constitutes the K+ ion selectivity filter of the channel which is characterized by the signature sequence Gly-Tyr-Gly (57,58). This sequence allows stringent passage of the larger K+ ions through the channel into the cell and prevents the entry of smaller, more abundant Na+ ions (59). Immunohistochemical studies have shown that GIRK4 is localized mainly to the ZG of the human adrenal cortex and to the outer part of the ZF (40,53,60). GIRK4 and other K+ channels maintain the hyperpolarized state of the ZG cell by allowing an outward flow of K+ conductance (54,55) (Figure 3).
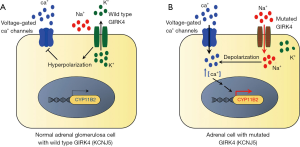
The advent of large-scale methods of analyses such as gene sequencing [e.g., next generation sequencing (NGS) and whole exome sequencing] in the last decade has helped to elucidate the genetic landscape and molecular mechanism of PA pathogenesis. The genetic basis of PA was largely an uncharted territory until Choi et al. used exome sequencing to first report a role for somatic mutations in driving autonomous aldosterone production in APA in 2011 (53). This study identified two “hot-spot” somatic mutations (p.Gly151Arg (G151R) and p.Leu168Arg (L168R) substitutions) in KCNJ5 (53) (Figure 4). These originally described KCNJ5 somatic gain-of-function mutations were located near or within the selectivity filter and disrupt its ion selectivity by facilitating indiscriminate entry of Na+ through the pore of the outer tunnel (53). The resulting depolarization of the cell membrane induces the opening of the voltage-gated Ca2+ channels leading to elevated intracellular Ca2+, increased activation of the calcium signaling pathway, augmented CYP11B2 transcription and aldosterone biosynthesis (Figure 3). Two cell-based analyses from our group established that mutated KCNJ5 activates the acute and chronic regulatory steps of aldosterone production and that increased aldosterone production occurs along with elevations in CYP11B2, and its regulatory transcription factors nuclear receptor related 1 protein (NURR1) and activating transcription factor 2 (ATF2) (47,60). Besides G151R and L168R which constitute 90% of all the KCNJ5 APA mutations, 24 other KCNJ5 mutations have also been detected in APA (40,41,53,61-70,74-77) (Figure 4).
Along with their studies on the genetic causes of somatic APA, Choi et al. also established the genetic basis of FH type III by identifying a novel gain-of-function KCNJ5 germline mutation in a father and his two daughters, all with PA (53). This substitution mutation—p.Thr158Ala (T158A)—is located near the selectivity filter of the channel pore. The T158A mutation was later also detected in sporadic APA by Mulatero et al. in 2012 (76). Adrenocortical cell line studies demonstrated that the p.Thr158Ala mutation in KCNJ5 causes an increase in aldosterone production via membrane depolarization and Na+ and Ca2+ influx (47,48). While patients carrying the germline mutations G151R (similar to the recurrent somatic KCNJ5 mutation in APA), T158A and p.Ile157Ser, presented with early onset and a severe PA phenotype with drug-resistant hypertension and adrenal hyperplasia (71,72), those with the p.Gly151Glu (G151E) (72) and p.Tyr152Cys (73) variants exhibited a remarkably milder phenotype.
Somatic KCNJ5 mutations in APA—the demographics
The last 10 years have resulted in a plethora of studies in the field of PA that have investigated the presence and racial prevalence of the various aldosterone-driving somatic mutations in APA, including KCNJ5. Collaborating investigators from the European Network for the Study of Adrenal Tumors (ENS@T) have conducted the largest mutation prevalence studies to date assessing 474 APA with the sequencing directed at the previously reported mutation hotspots (39). This multicenter study demonstrated the presence of somatic mutations in 54% of APA, with genetic abnormalities in KCNJ5 representing 38% (39). This study corroborated the frequency of somatic mutations in KCNJ5 that were identified in previous smaller studies in European populations (35,74,75,78,79). Of note, the prevalence of reported somatic mutations in APA has been shown to vary by race. In particular, somatic KCNJ5 mutations are much more common in East Asian patients than in Europeans (70% vs. 38%) (36,49,50,65,68,80-82). We recently conducted two studies wherein we determined the incidence of APA somatic mutations in White Americans and Americans of African descent (Blacks) (41,77). Instead of using grossly dissected snap frozen tumor tissue as was done in previous studies, we applied a unique sequencing approach by utilizing a CYP11B2 immunohistochemistry (IHC)-guided comprehensive NGS protocol targeting the entire coding regions of a panel of genes frequently mutated in APA. While the KCNJ5 gene aberrations were most frequently seen in White Americans (43%) (41), Blacks showed a different APA somatic mutation profile with KCNJ5 (34%) being the second-most mutated gene after CACNA1D (77). Notably, the unique approach of utilizing the CYP11B2 IHC-directed adenoma selection and a comprehensive, full coding sequence-based NGS approach overturned the previously published prevalence by demonstrating that over 90% of APA have a known aldosterone-driver gene. However, in agreement with previous studies that utilized non-IHC-directed, selected exon sequencing‒based approaches, aldosterone-driving mutations were not identified in CYP11B2-negative tumor samples by the IHC-guided method (41,77,83,84). Collectively, this suggests that the heterogeneity of CYP11B2 expression in PA adrenals could have led to discrepancies between macroscopic-guided sequencing and the CYP11B2 IHC-guided sequencing results.
While KCNJ5 mutations in APA have consistently been reported to be more frequent in females in Western populations (35,39,41,74,75,77-79), this distinction was not clear in East Asians where the prevalence was very high in both men and women (36,49,50,65,68,80-82,85). The association of KCNJ5 mutations with sex (women higher than men), younger age, pronounced hyperaldosteronism and larger tumor size was also demonstrated in a meta-analysis study comprising 1,636 APA patients from 13 studies (86).
Conclusions and perspectives
The current diagnostic work-up for PA is a complex multitiered process (10). Although the Endocrine Society Guidelines recommend measurement of the aldosterone-to-renin ratio (ARR) as an appropriate initial screening test for PA, ARR is only about 80% specific and sensitive (87-89). Furthermore, it requires confirmatory testing of aldosterone production following sodium loading and volume expansion (10). More importantly, the ARR is elevated in patients with both BHA and APA. Consequently, the biochemical diagnosis of PA does not differentiate between the two primary causes of PA, and follow-up procedures are needed to classify patients with PA. The major impediments to screening for and treating PA are the complexities in the later stages of the evaluation. Currently available laboratory tests can confirm the diagnosis of PA, but neither these tests, nor current imaging studies can determine which adrenal gland(s) is (are) the source(s) of aldosterone. There is general agreement that cross-sectional imaging studies such as computed tomography scanning or magnetic resonance imaging cannot distinguish between most APA and BHA cases. This failure derives from both the small size of most APA and the high prevalence of nonfunctional adrenocortical adenomas in the general population, most of which do not produce aldosterone. Thus, the expensive, technically cumbersome and invasive AVS technique remains the gold standard for the subtyping of patients with PA. The variable success rates of AVS methodology from center-to-center has prompted researchers to consider alternative non-invasive diagnostic tools that could aid in diagnosing and classifying the different forms of PA.
Recent advances in the diagnostics of PA have been possible with the emergence of the LC-MS/MS methodology which allows steroid profiling in an individual patient. Increasing evidence suggests that steroid fingerprinting might be a major determinant in not only differentiating APA from BHA, but also in providing genotype-phenotype associations of APA, thereby making it a useful tool in simplifying the complex diagnostic work-up in patients with suspected PA. The utility of hybrid steroids—18OHF and 18oxoF—as potential differentiators between APA and BHA, has been tested for the past three decades with encouraging results. In fact, a panel of 12 steroids including the hybrid steroids in peripheral plasma that was put forth by Eisenhofer et al. was successful in classifying 80% of the PA cases as APA or BHA (24). The same research group also demonstrated that the putative application of steroid profiling for subtype classification of PA is likely due to the association of the steroid metabolome with somatic APA aldosterone-driver mutations (44). Notably, they showed that the high levels of hybrid steroids could be a signature for KCNJ5-mutated APA. Moreover, steroid profiling of peripheral blood was able to correctly categorize 92% of the somatic APA mutations. They recently took a similar diagnostic route in the case of a 55-year-old female patient with left adrenal mass in whom AVS was a failure (90). The patient’s peripheral plasma displayed increased levels of aldosterone, 18OHF, 18oxoF, 11-DOC, 11-deoxycortisol (90) which were indicative of a macro-APA as a result of a KCNJ5 mutation (45). Adrenalectomy was recommended for this patient and the diagnosis was later confirmed by genetic testing and histopathology (90). This example highlights the clinical applicability of steroid fingerprinting in the PA work-up.
The inclusion of steroid profiling in the PA diagnostic chart has several advantages. Sixty percent to 70% of patients with PA have BHA and thus receive no benefit from the AVS procedure, because they are treated medically rather than surgically. Certain populations would benefit from the new diagnostic approach following ARR wherein steroid profiling could help identify patients that need to undergo the CT and AVS and bypass invasive testing in patients with BHA, who require lifelong personalized medical treatment. KCNJ5 mutations, which are more prevalent in women and constitute the majority of East Asian APA cases of both sexes, could be diagnosed rapidly by serum hybrid steroids. Given the high prevalence of KCNJ5 mutations in PA patients, macrolides might potentially be used to identify patients that bear APA with KCNJ5 mutations (91) and as targeted personalized treatments for these patients. Inclusion of steroid fingerprinting in the PA diagnostic work-up will reduce healthcare cost and increase the impact on patient safety for almost 60–70% PA patients by reducing radiation exposure and application of invasive procedures. Prospective studies will, however, be needed to validate whether this method is a better alternative to the invasive and technically onerous AVS.
Acknowledgments
Funding: This work was supported by grants K08 DK109116 A.F.T and R01 DK106618 and R01 DK043140 to W.E.R. from the National Institutes of Health (NIH), and grant 2019087 from the Doris Duke Charitable Foundation to AFT.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf) 2007;66:607-18. [Crossref] [PubMed]
- Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006;48:2293-300. [Crossref] [PubMed]
- Fardella CE, Mosso L, Gomez-Sanchez C, et al. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab 2000;85:1863-7. [PubMed]
- Gordon RD, Stowasser M, Tunny TJ, et al. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharmacol Physiol 1994;21:315-8. [Crossref] [PubMed]
- Lim PO, Dow E, Brennan G, et al. High prevalence of primary aldosteronism in the Tayside hypertension clinic population. J Hum Hypertens 2000;14:311-5. [Crossref] [PubMed]
- Loh KC, Koay ES, Khaw MC, et al. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. J Clin Endocrinol Metab 2000;85:2854-9. [PubMed]
- Calhoun DA, Nishizaka MK, Zaman MA, et al. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002;40:892-6. [Crossref] [PubMed]
- Strauch B, Zelinka T, Hampf M, et al. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens 2003;17:349-52. [Crossref] [PubMed]
- Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 2008;371:1921-6. [Crossref] [PubMed]
- Funder JW, Carey RM, Mantero F, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016;101:1889-916. [Crossref] [PubMed]
- Mulatero P, Tizzani D, Viola A, et al. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (Primary Aldosteronism in TOrino-GENetic forms). Hypertension 2011;58:797-803. [Crossref] [PubMed]
- Young WF Jr. Minireview: primary aldosteronism--changing concepts in diagnosis and treatment. Endocrinology 2003;144:2208-13. [Crossref] [PubMed]
- Else T, Williams AR, Sabolch A, et al. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab 2014;99:455-61. [Crossref] [PubMed]
- Monticone S, Burrello J, Tizzani D, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol 2017;69:1811-20. [Crossref] [PubMed]
- Prada ETA, Burrello J, Reincke M, et al. Old and New Concepts in the Molecular Pathogenesis of Primary Aldosteronism. Hypertension 2017;70:875-81. [Crossref] [PubMed]
- Lenders JWM, Eisenhofer G, Reincke M. Subtyping of Patients with Primary Aldosteronism: An Update. Horm Metab Res 2017;49:922-8. [Crossref] [PubMed]
- Monticone S, Viola A, Rossato D, et al. Adrenal vein sampling in primary aldosteronism: towards a standardised protocol. Lancet Diabetes Endocrinol 2015;3:296-303. [Crossref] [PubMed]
- Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014;63:151-60. [Crossref] [PubMed]
- Stowasser M, Bachmann AW, Tunny TJ, et al. Production of 18-oxo-cortisol in subtypes of primary aldosteronism. Clin Exp Pharmacol Physiol 1996;23:591-3. [Crossref] [PubMed]
- Satoh F, Morimoto R, Ono Y, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension 2015;65:1096-102. [Crossref] [PubMed]
- Ulick S, Blumenfeld JD, Atlas SA, et al. The unique steroidogenesis of the aldosteronoma in the differential diagnosis of primary aldosteronism. J Clin Endocrinol Metab 1993;76:873-8. [PubMed]
- Ulick S, Chu MD, Land M. Biosynthesis of 18-oxocortisol by aldosterone-producing adrenal tissue. J Biol Chem 1983;258:5498-502. [PubMed]
- Mulatero P, di Cella SM, Monticone S, et al. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab 2012;97:881-9. [Crossref] [PubMed]
- Eisenhofer G, Dekkers T, Peitzsch M, et al. Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism. Clin Chem 2016;62:514-24. [Crossref] [PubMed]
- Nakamura Y, Satoh F, Morimoto R, et al. 18-oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J Clin Endocrinol Metab 2011;96:E1272-8. [Crossref] [PubMed]
- Gomez-Sanchez CE, Montgomery M, Ganguly A, et al. Elevated urinary excretion of 18-oxocortisol in glucocorticoid-suppressible aldosteronism. J Clin Endocrinol Metab 1984;59:1022-4. [Crossref] [PubMed]
- Gordon RD, Hamlet SM, Tunny TJ, et al. Aldosterone-producing adenomas responsive to angiotensin pose problems in diagnosis. Clin Exp Pharmacol Physiol 1987;14:175-9. [Crossref] [PubMed]
- Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension 2003;42:161-5. [Crossref] [PubMed]
- Mosso L, Gomez-Sanchez CE, Foecking MF, et al. Serum 18-hydroxycortisol in primary aldosteronism, hypertension, and normotensives. Hypertension 2001;38:688-91. [Crossref] [PubMed]
- Gomez-Sanchez CE, Upcavage RJ, Zager PG, et al. Urinary 18-hydroxycortisol and its relationship to the excretion of other adrenal steroids. J Clin Endocrinol Metab 1987;65:310-4. [Crossref] [PubMed]
- Lifton RP, Dluhy RG, Powers M, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 1992;355:262-5. [Crossref] [PubMed]
- Gomez-Sanchez CE, Clore JN, Estep HL, et al. Effect of chronic adrenocorticotropin stimulation on the excretion of 18-hydroxycortisol and 18-oxocortisol. J Clin Endocrinol Metab 1988;67:322-6. [Crossref] [PubMed]
- Lenders JWM, Williams TA, Reincke M, et al. DIAGNOSIS OF ENDOCRINE DISEASE: 18-Oxocortisol and 18-hydroxycortisol: is there clinical utility of these steroids? Eur J Endocrinol 2018;178:R1-R9. [Crossref] [PubMed]
- Ulick S, Chan CK, Gill JR Jr, et al. Defective fasciculata zone function as the mechanism of glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 1990;71:1151-7. [Crossref] [PubMed]
- Azizan EAB, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 2013;45:1055-60. [Crossref] [PubMed]
- Kitamoto T, Suematsu S, Yamazaki Y, et al. Clinical and Steroidogenic Characteristics of Aldosterone-Producing Adenomas With ATPase or CACNA1D Gene Mutations. J Clin Endocrinol Metab 2016;101:494-503. [Crossref] [PubMed]
- Monticone S, Castellano I, Versace K, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol 2015;411:146-54. [Crossref] [PubMed]
- Yamazaki Y, Omata K, Tezuka Y, et al. Tumor Cell Subtypes Based on the Intracellular Hormonal Activity in KCNJ5-Mutated Aldosterone-Producing Adenoma. Hypertension 2018;72:632-40. [Crossref] [PubMed]
- Fernandes-Rosa FL, Williams TA, Riester A, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 2014;64:354-61. [Crossref] [PubMed]
- Azizan EA, Lam BY, Newhouse SJ, et al. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab 2012;97:E819-29. [Crossref] [PubMed]
- Nanba K, Omata K, Else T, et al. Targeted Molecular Characterization of Aldosterone-Producing Adenomas in White Americans. J Clin Endocrinol Metab 2018;103:3869-76. [Crossref] [PubMed]
- Azizan EA, Brown MJ. Novel genetic determinants of adrenal aldosterone regulation. Curr Opin Endocrinol Diabetes Obes 2016;23:209-17. [Crossref] [PubMed]
- Mulatero P, Curnow KM, Aupetit-Faisant B, et al. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J Clin Endocrinol Metab 1998;83:3996-4001. [PubMed]
- Williams TA, Peitzsch M, Dietz AS, et al. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension 2016;67:139-45. [Crossref] [PubMed]
- Yang Y, Burrello J, Burrello A, et al. Classification of microadenomas in patients with primary aldosteronism by steroid profiling. J Steroid Biochem Mol Biol 2019;189:274-82. [Crossref] [PubMed]
- Murakami M, Rhayem Y, Kunzke T, et al. In situ metabolomics of aldosterone-producing adenomas. JCI Insight 2019;4. [Crossref] [PubMed]
- Hattangady NG, Karashima S, Yuan L, et al. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J Mol Endocrinol 2016;57:1-11. [Crossref] [PubMed]
- Oki K, Plonczynski MW, Luis Lam M, et al. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology 2012;153:1774-82. [Crossref] [PubMed]
- Kitamoto T, Suematsu S, Matsuzawa Y, et al. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb 2015;22:191-200. [Crossref] [PubMed]
- Okamura T, Nakajima Y, Katano-Toki A, et al. Characteristics of Japanese aldosterone-producing adenomas with KCNJ5 mutations. Endocr J 2017;64:39-47. [Crossref] [PubMed]
- Tezuka Y, Yamazaki Y, Kitada M, et al. 18-Oxocortisol Synthesis in Aldosterone-Producing Adrenocortical Adenoma and Significance of KCNJ5 Mutation Status. Hypertension 2019;73:1283-90. [Crossref] [PubMed]
- Geller DS, Zhang J, Wisgerhof MV, et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 2008;93:3117-23. [Crossref] [PubMed]
- Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011;331:768-72. [Crossref] [PubMed]
- Mulatero P, Monticone S, Rainey WE, et al. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol 2013;9:104-12. [Crossref] [PubMed]
- Williams TA, Lenders JW, Burrello J, et al. KCNJ5 Mutations: Sex, Salt and Selection. Horm Metab Res 2015;47:953-8. [Crossref] [PubMed]
- Krapivinsky G, Gordon EA, Wickman K, et al. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature 1995;374:135-41. [Crossref] [PubMed]
- Gomez-Sanchez CE, Oki K. Minireview: potassium channels and aldosterone dysregulation: is primary aldosteronism a potassium channelopathy? Endocrinology 2014;155:47-55. [Crossref] [PubMed]
- Hibino H, Inanobe A, Furutani K, et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 2010;90:291-366. [Crossref] [PubMed]
- Bichet D, Haass FA, Jan LY. Merging functional studies with structures of inward-rectifier K(+) channels. Nat Rev Neurosci 2003;4:957-67. [Crossref] [PubMed]
- Monticone S, Hattangady NG, Nishimoto K, et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab 2012;97:E1567-72. [Crossref] [PubMed]
- Murthy M, Azizan EA, Brown MJ, et al. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J Hypertens 2012;30:1827-33. [Crossref] [PubMed]
- Cheng CJ, Sung CC, Wu ST, et al. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3.4 membrane abundance. J Clin Endocrinol Metab 2015;100:E155-63. [Crossref] [PubMed]
- Hardege I, Xu S, Gordon RD, et al. Novel Insertion Mutation in KCNJ5 Channel Produces Constitutive Aldosterone Release From H295R Cells. Mol Endocrinol 2015;29:1522-30. [Crossref] [PubMed]
- Scholl UI, Healy JM, Thiel A, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf) 2015;83:779-89. [Crossref] [PubMed]
- Wang B, Li X, Zhang X, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore) 2015;94:e708. [Crossref] [PubMed]
- Zheng FF, Zhu LM, Zhou WL, et al. A novel somatic mutation 145-147delETEinsK in KCNJ5 increases aldosterone production. J Hum Hypertens 2017;31:756-9. [Crossref] [PubMed]
- Nanba K, Omata K, Tomlins SA, et al. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur J Endocrinol 2016;175:K1-6. [Crossref] [PubMed]
- Zheng FF, Zhu LM, Nie AF, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension 2015;65:622-8. [Crossref] [PubMed]
- Kuppusamy M, Caroccia B, Stindl J, et al. A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J Clin Endocrinol Metab 2014;99:E1765-73. [Crossref] [PubMed]
- Kitamoto T, Omura M, Suematsu S, et al. KCNJ5 mutation as a predictor for resolution of hypertension after surgical treatment of aldosterone-producing adenoma. J Hypertens 2018;36:619-27. [Crossref] [PubMed]
- Charmandari E, Sertedaki A, Kino T, et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab 2012;97:E1532-9. [Crossref] [PubMed]
- Scholl UI, Nelson-Williams C, Yue P, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci U S A 2012;109:2533-8. [Crossref] [PubMed]
- Monticone S, Hattangady NG, Penton D, et al. a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab 2013;98:E1861-5. [Crossref] [PubMed]
- Williams TA, Monticone S, Schack VR, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension 2014;63:188-95. [Crossref] [PubMed]
- Åkerström T, Crona J, Delgado Verdugo A, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One 2012;7:e41926. [Crossref] [PubMed]
- Mulatero P, Tauber P, Zennaro MC, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension 2012;59:235-40. [Crossref] [PubMed]
- Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension 2019;73:885-92. [Crossref] [PubMed]
- Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 2013;45:440-4, 4e1-2.
- Boulkroun S, Beuschlein F, Rossi GP, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension 2012;59:592-8. [Crossref] [PubMed]
- Wu VC, Wang SM, Chueh SJ, et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep 2017;7:39121. [Crossref] [PubMed]
- Warachit W, Atikankul T, Houngngam N, et al. Prevalence of Somatic KCNJ5 Mutations in Thai Patients With Aldosterone-Producing Adrenal Adenomas. J Endocr Soc 2018;2:1137-46. [Crossref] [PubMed]
- Hong AR, Kim JH, Song YS, et al. Genetics of Aldosterone-Producing Adenoma in Korean Patients. PLoS One 2016;11:e0147590. [Crossref] [PubMed]
- Nanba K, Chen AX, Omata K, et al. Molecular Heterogeneity in Aldosterone-Producing Adenomas. J Clin Endocrinol Metab 2016;101:999-1007. [Crossref] [PubMed]
- Dekkers T, ter Meer M, Lenders JW, et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab 2014;99:E1341-51. [Crossref] [PubMed]
- Wu VC, Huang KH, Peng KY, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep 2015;5:11396. [Crossref] [PubMed]
- Lenzini L, Rossitto G, Maiolino G, et al. A Meta-Analysis of Somatic KCNJ5 K(+) Channel Mutations In 1636 Patients With an Aldosterone-Producing Adenoma. J Clin Endocrinol Metab 2015;100:E1089-95. [Crossref] [PubMed]
- Rossi GP, Barisa M, Belfiore A, et al. The aldosterone-renin ratio based on the plasma renin activity and the direct renin assay for diagnosing aldosterone-producing adenoma. J Hypertens 2010;28:1892-9. [Crossref] [PubMed]
- Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 2004;89:1045-50. [Crossref] [PubMed]
- Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3266-81. [Crossref] [PubMed]
- Holler F, Heinrich DA, Adolf C, et al. Steroid Profiling and Immunohistochemistry for Subtyping and Outcome Prediction in Primary Aldosteronism-a Review. Curr Hypertens Rep 2019;21:77. [Crossref] [PubMed]
- Scholl UI, Abriola L, Zhang C, et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J Clin Invest 2017;127:2739-50. [Crossref] [PubMed]