Robotic transaxillary thyroidectomy: state of the art
Introduction
Although robot-assisted transaxillary thyroidectomy (RATT) has become a widely used treatment modality for thyroid disease in the Far East, the procedure remains under discussion in Western World (1,2). Different body mass index, anthropometric characteristics, size of tumours, goitre and ethical considerations, combined with elevated cost of the procedure and the need of training, hinder the diffusion of this approach in US and Europe (1,3). To date, RATT, although excellent results in terms of feasibility, oncological safety and patient’s compliance are described, plays a niche role in selected patients with appropriate pathology in high-volume centers (1,4).
Indications for RATT varies among the centers, but nowadays substantially both benign pathology and well-differentiated low risk thyroid carcinoma can be approached with the robotic technique. Usually, previous neck or breast surgery are considered robotic controindications, as well as neck radiotherapy, pacemaker implant in the major pectoralis region in case of necessity of same side axillary access, shoulder arthrosis, and previous shoulder surgery (4).
Concerning oncological safety, it is well described in literature by several systematic reviews and meta-analyses that there are not significant differences between the robotic and open techniques in terms of loco-regional recurrence and survival rates, serum thyroglobulin levels, post-ablation radioactive iodine scan uptake and lymph node yield. These data suggest that robotic thyroidectomy is comparable to open thyroidectomy in terms of oncological safety when performed by experienced surgeons (1,5).
Surgical procedure
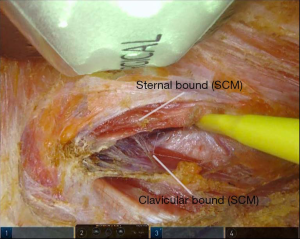
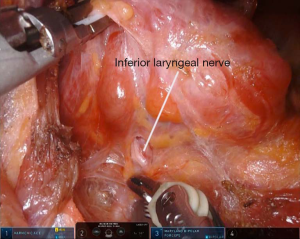
The operation begins with a 5–6 cm incision along the posterior border of the right anterior axillary pillar. The dissection of the skin flap is performed by using a monopolar scalpel. The surgical space is maintained using a retractor which elevates a subcutaneous flap above the pectoralis major muscle and whose position is changed and adapted during dissection. Further, the access to the thyroid lodge is reached passing through the two heads of the sternocleidomastoid muscle (SCM) (sternal and clavicular) (Figure 1). We proceed with a blunt dissection of the strap muscles. Positioning of a specific robotic-thyroidectomy retractor in order to maintain the surgical space. The moment of Da Vinci Xi® robot docking is come. In our institutions, only three robotic arms are used and the fourth is kept folded. Trans-incision positioning of a 3D camera, a harmonic scalpel and a Maryland dissector, at the middle point, at the cranial vertex and at the lower vertex, respectively. Anyway, other centers use four robotic instrument, with the latter, a Prograsper, positioned under the superior edge of the incision or through a small incision in the axillary region or at the breast level. From this moment forward, the procedure is performed from the robotic console, while an assistant, at the operating table, provides suction and counter-traction when required. We cut the upper pedicle with harmonic scalpel and subsequently we cut the thyroid isthmus. After the identification of the inferior laryngeal nerve and the ipsilateral parathyroid glands (Figure 2), the thyroid lobe is removed following the tracheal plane, respecting the noble structures. The thyroid lobe is extracted by using an endobag to avoid potential malignant seeding along the incision. In case total thyroidectomy is required, the contralateral lobe is removed through the same surgical access (2,6).
Discussion
Drawbacks and advantages
Drawbacks
To date, RATT presents some limitations, most of them proper of robotic surgery. Firstly, the loss of haptic feedback that could lead to breaking the suture thread during knot tying or to tear the tissue during handling. Nonetheless, these drawbacks are rare in thyroid surgery and mostly represented in abdominal surgery or in intervention which requests reconstructive steps. Anyway, the loss of haptic feedback is progressively compensated by most surgeons with the increase of experience and thanks to availability of a 3D magnified vision that allows fine movements (7). The cumbersome nature of the robotic platform, which require supplementary space and time for connecting the instruments and for the organisation of the sterile envelope, represents a second drawback. Regarding the operative time of RATT—almost double compared with open thyroidectomy—it is important to take into consideration that it tends to decrease with the accumulation of experience and arrives at a steady state after 35 to 40 cases, a number considerably lower compared to the learning curve of the endoscopic transaxillary procedure (55 to 60 cases) (2,7). Moreover, in some high-volume centers, the operating time is down cast even positioning only 3 robotic arms and keeping the fourth folded: this tip decreases robotic arms collision allowing the surgeon more fluid movements (2). Last but not least, the exceeded operating time compared to open thyroidectomy does not lead to alterations of outcomes, quality of life or length of hospital stay (1).
In terms of cost, RATT is a more expensive procedure than open thyroidectomy due to the prohibitive cost of the instruments and their maintenance and the longer operative time (8,9). Anyhow, some studies underlined that RATT does not require the presence of a third surgical assistant, and, as stated above, the steep learning curve decreases progressively the operative time, both factors that conduced to break down the cost (1-8). Moreover, with the imminent entry of medical device companies in the surgical robot field, competition can be expected to decrease the cost (1). Finally, by centralising robotic thyroidectomy performance to high-volume centers, further reduction of the cost can be obtained, as well as better surgical outcomes (1,4,10).
RATT introduces potential new complications, such as brachial plexus neuropathies, chest pain and paraesthesia (6). The chance of the first one should be avoided placing the arm in a flexed position, avoiding more than 90 degrees of extension on the elbow and shoulder joints (10). Further, intra-operative axillary nerve monitoring may potentially reduce the possibility of brachial plexus injury and enabling the patient to be repositioned (8). Regarding the latter, it is reported that increased skin flap dissection of RATT does not translate to increased pain and paraesthesia: on the contrary, Ryu et al. reported less post-operative overall pain in patients who underwent RATT compared to whom underwent traditional thyroidectomy (1,11,12).
Lastly, it is important to take into consideration the risk—although very low—of seeding of tumour cells along the incision. This kind of risk is usually related to a fractured specimen, but this complication can occur even without fragmented resection, especially in the presence of large malignant nodule, extra thyroidal extension and perineural invasion (13). For this reason, we suggest the routine use of an adequate-size endobag for removing the specimen.
Advantages
The most evident benefit of RATT over conventional open thyroidectomy is the absence of any cervical scar. For this cosmetic profit, RATT is endearing especially for young female patients or for those with a tendency toward keloid formation (8).
Moreover, RATT presented several other technical advantages over the conventional open and endoscopic thyroidectomy. The robotic system provides a magnified 3D vision that allows a simpler identification of the noble structures neighbouring to the thyroid gland (recurrent laryngeal nerve and parathyroid glands). Further, the tremor-filtering system, the endowrist technology and the multi-articulations of the arms (7 degrees of freedom) allow wider and finer movements (4,8,11). This aspect may reduce the interference between instruments and robotic camera and allows avoiding annoying changing in the orientation of the direction of the view even when the instruments converge parallel to the surgeon’s eye (14).
These features allow to overcoming some limitations such as the use of robotic arms in a deep and narrow space containing fragile organs surrounded by critical nerves and major vessels (11). As result, with the robotic approach, the identification and the manipulation of the noble structures allocated in the confined space of the neck is made easier, resulting in minimal complication rates, optimum oncologic outcomes and patient’s satisfaction (1,8). Besides, there are reported in literature cases of faster recovery of voice and swallowing function with the robotic approach, compared to open thyroidectomy (5).
Finally, it is not negligible that RATT is performed by surgeon comfortably sitting at console: this aspect translates in a higher postoperative ergonomic satisfaction (15).
Robotic neck dissection
RATT has been extended even to cervical lymph node dissection in selected cases, proving that robotic technology can overwhelm some technical limits of endoscopic approach (7). The precise dexterity of robotic system with magnified 3D vision enable an accurate and safe dissection with a number of removed lymph nodes comparable to whom of open surgery (8).
In comparing the oncologic outcomes, safety and quality of life of patients with similar clinical and pathological characteristics who underwent either robotic or open total thyroidectomy with modified radical neck dissection, it is reported that the first approach was as effective and safe as conventional open surgery (5,15). Furthermore, this technique could give additional benefits for quality of life and patient’s satisfaction, such as reducing sensory alterations in the neck and allowing an early and adequate swallow, as well as excellent cosmesis (5).
Conclusions
RATT is feasible and oncological safe in selected cases and allows avoiding a visible neck scar.
Robotic approach, through a more precise movement system and a tremor-filtering technology lead to optimum outcomes in term of complications rate compared to open or endoscopic surgery. For surgeons, RATT results in less musculoskeletal discomfort and more ergonomic satisfaction.
Capacity can be improved with a steep learning curve in a relatively short time, with an associated low complication rate. In the future, with the entry of new medical device companies in the surgical robot field, the development of technologies, and the spreading of the procedure, some limitations, such as the lack of haptic feedback, the cumbersome of robotic instruments, the elevated operative time and the prohibitive cost will be gradually improved.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Aidan P, Arora A, Lorincz B, et al. Robotic Thyroid Surgery: Current Perspectives and Future Considerations. ORL J Otorhinolaryngol Relat Spec 2018;80:186-94. [Crossref] [PubMed]
- Materazzi G, Fregoli L, Papini P, et al. Robot-Assisted Transaxillary Thyroidectomy (RATT): A Series Appraisal of More than 250 Cases from Europe. World J Surg 2018;42:1018-23. [Crossref] [PubMed]
- Fortuny JV, Guigard S, Karenovics W, et al. Surgery of the thyroid: recent developments and perspective. Swiss Med Wkly 2015;145:w14144. [PubMed]
- Piccoli M, Mullineris B, Gozzo D, et al. Evolution Strategies in Transaxillary Robotic Thyroidectomy: Considerations on the First 449 Cases Performed. J Laparoendosc Adv Surg Tech A 2019;29:433-40. [Crossref] [PubMed]
- Kang SW, Kim MJ, Chung WY. Gasless, transaxillary robotic neck dissection: the technique and evidence. Gland Surg 2018;7:466-72. [Crossref] [PubMed]
- Prete FP, Marzaioli R, Lattarulo S, et al. Transaxillary robotic-assisted thyroid surgery: technique and results of a preliminary experience on the Da Vinci Xi platform. BMC Surg 2019;18:19. [Crossref] [PubMed]
- Fan LJ, Jiang J. Present and future of robot-assisted endoscopic thyroid surgery. Chin Med J (Engl) 2012;125:926-31. [PubMed]
- Rabinovics N, Feinmesser R, Aidan P, et al. Robot-assisted transaxillary thyroid surgery-feasibility and safety of a novel technique. Rambam Maimonides Med J 2014;5:e0013. [Crossref] [PubMed]
- Cabot JC, Lee CR, Brunaud L, et al. Robotic and endoscopic transaxillary thyroidectomies may be cost prohibitive when compared to standard cervical thyroidectomy: a cost analysis. Surgery 2012;152:1016-24. [Crossref] [PubMed]
- Zaidi N, Daskalaki D, Quadri P, et al. The current status of robotic transaxillary thyroidectomy in the United States: an experience from two centers. Gland Surg 2017;6:380-4. [Crossref] [PubMed]
- Ryu HR, Lee J, Park JH, et al. A comparison of postoperative pain after conventional open thyroidectomy and transaxillary single-incision robotic thyroidectomy: a prospective study. Ann Surg Oncol 2013;20:2279-84. [Crossref] [PubMed]
- Arora A, Garas G, Sharma S, et al. Comparing transaxillary robotic thyroidectomy with conventional surgery in a UK population: A case control study. Int J Surg 2016;27:110-7. [Crossref] [PubMed]
- Chabrillac E, Zerdoud S, Fontaine S, et al. Multifocal recurrence on the transaxillary robotic thyroidectomy incision. Eur Ann Otorhinolaryngol Head Neck Dis 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Chang YW, Lee HY, Ji WB, et al. Detailed comparison of robotic and endoscopic transaxillary thyroidectomy. Asian J Surg 2019. [Epub ahead of print]. [PubMed]
- Lee J, Chung WY. Robotic thyroidectomy and neck dissection: past, present, and future. Cancer J 2013;19:151-61. [Crossref] [PubMed]