Subxiphoid thymectomy with a double sternum retractor: a pilot study
Introduction
The performance of thymectomies via the subxiphoid approach was first introduced by Kido et al. in 1999 (1,2). Since then, many new aspects of this approach have been introduced, such as the lifting of the sternum through CO2 insufflation and a sternum retractor (3). Because a camera is inserted through the midline subxiphoid region, the operative view of the neck region and locations of the bilateral intercostal nerves are easily accessible, particularly with the help of a sternum retractor (1). In particular, the subxiphoid approach is an excellent technique for both surgeons and patients because the operative field in the neck region is secured, bilateral phrenic nerve identification is possible, cosmetic outcomes are superior, and pain is minimal (4). The introduction of the double hook before the operation is critical to creating more space for the surgeon. One hook is usually placed at the level of the manubrium and one at the level of the subxiphoid process.
In our pilot study, we enrolled 34 patients who underwent thymectomy using the subxiphoid approach for an anterior mediastinal benign or resectable malignant tumor, and double sternum hooks were used to improve our vision of the anatomical structures and create a better space during resection. This study aims to determine the safety and usefulness of the subxiphoid approach associated with the use of sternum retractors.
Methods
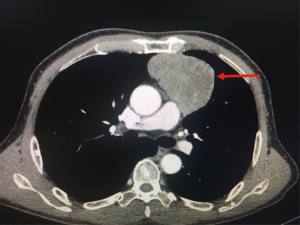
From November 2016 until July 2018, we enrolled 34 patients who underwent subxiphoid thymectomy with a double sternum retractor in our Thoracic Surgery Department. The Ethics Board Committee approved the study, and each patient provided written informed consent before enrollment. The Masaoka staging system was adopted as commonly used for thymomas (5), in particular, stage I represented by intact thymic capsule, stage II represented by capsular invasion into adjacent mediastinal fat or pleura, stage III represented by macroscopic invasion into adjacent organs, vessels. Twenty of these patients were diagnosed with Masaoka Stage I–III thymomas, 12 with thymic hyperplasia or cysts of the thymus, and 2 with thymic tumors. All patients underwent a chest CT with enhancement (Figure 1). 18FDG-PET was performed in patients with thymomas or when recurrence was suspected. Anti-acetylcholine receptor (AChR) antibody levels were measured in all patients, and a neurological assessment was performed. Patients underwent subxiphoid thymectomy using a video-assisted thoracoscopic surgery (VATS) approach and the elevation of the sternum with a double retractor. A retrospective analysis of the clinical characteristics, operative data, postoperative events, such as the average pain score (NRS scale) (6,7) after surgery and average mental health and physical health scores (SF-12) (8), and follow-up was performed.
Operative technique
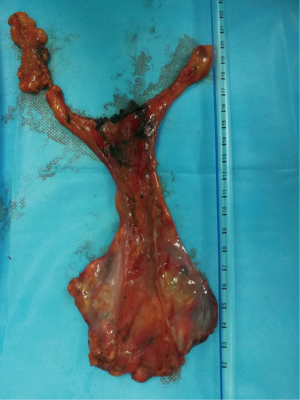
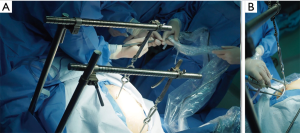
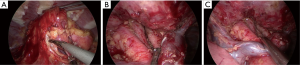
Subxiphoid thymectomy is performed in an intubated patient placed in the supine position. The operator stands between the legs and the surgical assistant to the right of the patient. A 4-cm vertical incision approximately 1-cm caudal to the xiphoid process and an additional 2-cm skin incision between the 4th and 5th intercostal anterior axillary line on the right side is usually performed (Figure 2). A 1-cm incision is generated to place hooks, which are tightened by two retractors: one at the level of the jugular process behind the manubrium of the sternum and the other one behind of the lower portion of the sternum after resection of the subxiphoid process, connected to a traction frame (Figure 3). The surgeon detaches the thymus and proceeds along the innominate vein by closing the thymic veins with vascular clips until the left internal thoracic vein is visualized. The dissection continues cranially with closure and division of the lower thyroid veins. The left lobe of the thymus is pulled toward the right side of the patient. At this point, the surgeon detaches the left lobe with an electronic device. The right lobe of the thymus is pulled towards the left side of the patient. The superficial adipose tissue is gradually detached from the area to expose the distal side of the left brachiocephalic vein safely. The neck portion is identified, and the thin membrane above the thymus is dissected. The superior pole of the thymus and the cervical adipose tissue are dissected from the right brachiocephalic vein on the right side, the thyroid at the upper end, the brachiocephalic artery and trachea on the posterior aspect, and the left brachiocephalic vein on the left side (Figure 4). Finally, the thymus is pulled to either the right or left and dissected from the innominate vein. All the fatty tissue around the thymus is removed to guarantee the cleanness of the margins. In sequential order, the thymic vein is dissected using the electronic device, and the thymectomy is completed (Figure 5).
Statistical analysis
Quantitative data are presented as medians and ranges, while qualitative data are presented as absolute and percentage frequencies. The incidence rates of mortality and complications were calculated as the number of events per 100 person-years. Patient pain was measured using a Numerical Rating Scale (NRS) ranging from 0 (no pain) to 10 (maximum pain) points (6,7), and quality of life was measured using the SF-12 questionnaire (8). Pain and quality of life were reported as average scores ± standard deviations.
Results
From November 2016 until July 2018, 34 patients were enrolled, with a median age of 56.5 years (range, 25–71 years); 12 (35.3%) were men and 22 (64.7%) women. Thymic neoformation ranged from 1.0 cm × 1.0 cm × 1.0 cm to 14.0 cm × 9.0 cm × 4.5 cm. PET-FDG showed hypermetabolism (SUV max >2.5) in 23 (69%) of patients. Neurological signs and the AChR antibody were positive in only 3 (15%) patients with thymomas. All patients underwent a subxiphoid thymectomy. The median operative time in all the stages considered was 2 hours (range, 1.3–4.0 hours), and intraoperative bleeding was 94 cc (range, 20–500 cc). A double hook was placed to elevate the sternum in all patients. Histology was positive in 20 patients with Masaoka Stage I–III thymomas, in 12 with thymic hyperplasia or cysts of the thymus and 2 with thymic tumors. No mortality or recurrence was observed after a median follow-up time of 17.9 months after surgery (range, 2.2–23.3 months). Complications occurred in 3 patients for a myasthenic crisis and two patients for diaphragmatic palsy. The mortality rate was zero, and the morbidity rate for complications was 8.8%. The average pain scores after surgery and after follow-up were 1.7±0.4 and 0.1±0.4, respectively, on the NRS scale. Average mental health and physical health scores after follow-up were 45.6±2.4 and 33.6±2.4, respectively, on the SF-12 scale.
Discussion
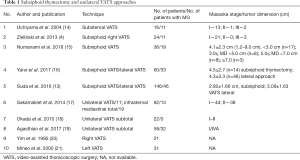
Although VATS provides clear benefits during thymus removal, it also has some disadvantages for the operator, such as the placement of long instruments through fixed entry points to create a fulcrum effect, bidimensional screen vision of the surgical field, and the creation of an unnatural visual effect that can cause the surgeon to lose the orientation and the eye-hand target axis when the camera is under an assistant’s control (9,10). Some robotic systems have been developed over the last two decades to overcome these limitations (11). However, few centers currently can use a robot, due to the costs (11,12). Subxiphoid thymectomy is considered a good compromise between robotic and VATS surgery to remove benign and no infiltrated malignant tumors, reducing the probability of intercostal nerve injuries (13). The benefits of this technique are the reduction in pain after surgery, the aesthetic result, and the lower costs. We strongly believe that the subxiphoid approach may be useful not only for removing small thymomas but also in treating more difficult selected cases. In particular, the use of double sternum retractors may be the best solution to visualize the mass at the level of the anterior mediastinum, particularly in patients with large tumors that infiltrate the tissue around the mass. The use of double sternum retractors provides the surgeon with a better view of the tumor, improving the surgical technique, and offers the possibility to remove the fat around the thymus altogether. This aspect is vital to ensure the radicality related to the surgical margins, although the VATS approach is limited and more static because the surgical instruments can only be manipulated in a limited space. This difficulty experienced during VATS surgery may affect the surgeon during the operation, reducing the concept of radicality. However, the subxiphoid approach is not yet considered the most common technique used to remove thymic masses because of one main disadvantage, the difficulty of this unusual uniportal approach. This technique not only relies on the experience of the surgeon but also the physical conformation of the patient. In some situations, obese patients and long-limbed patients may increase the difficulty of the surgery. Obese patients present with a small thorax, and double sternum retractors may be a beneficial instrument to create more space at the level of the thymic mass. We believe that extensive training is needed for surgeons to learn the highly specific technique of single-port surgery (9,13), particularly in the case of the subxiphoid approach. VATS subxiphoid thymectomy was developed many years ago and has continuously evolved from open to minimally invasive surgery, and in particular, from bilateral VATS to single port right or left VATS an ultimately to the monoportal subxiphoid approach (Table 1) (14-21). However, regarding the subxiphoid access, some operators regularly use two ports, one along the subxiphoid process and one between the 4th and 5th intercostal anterior axillary line on the right side for camera placement. This method improves the learning curve, reducing the risks of bleeding and intraoperative surgical complications. In 2017, Zieliński et al. reported the use of the hooks and described the technique of minimally invasive extended thymectomy performed through the uniportal subxiphoid approach for patients with nonthymomatous myasthenia gravis (MG) (22). In our case, we used the hooks for patients with thymomatous and nonthymomatous MG, as well as two patients with thymic carcinoma.
Full table
In summary, subxiphoid thymectomy could be extended to severe cases, such as large thymomas or thymic tumors infiltrating the pericardium or vessels; however, this approach should only be used in very selected cases, in high volume centers and by surgeons who are already well-trained in monoportal surgery with a high level of experience in this field. We strongly support the hypothesis that the use of the hooks in subxiphoid thymectomy is beneficial and facilitates the dissection by the surgeon, particularly in patients with large tumors where the visibility and the access of the anterior mediastinum are not guaranteed. Our experience obtained in this pilot study highlights the importance of the use of double sternum retractors in subxiphoid thymectomy for beginners and trained surgeons, particularly in challenging cases. This study proved the baseline for further investigations aiming to analyze the long-term results in a larger cohort of patients.
Acknowledgments
Funding: This Project has been funded by the National Natural Science Foundation of China (No. 81870008) and Shanghai Municipal Health Commission Special Program for Clinical Research in Health Industry (No. 201840175).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical Board Committee approved this study. Each patient signed a consent form to be enrolled.
References
- Shi Y, Sun F, Jin Y, et al. Subxiphoid approach with sternum retractor for mediastinal tumor cephalad to brachiocephalic vein. J Thorac Dis 2018;10:E473-E475. [Crossref] [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Takeo S, Sakada T, Yano T. Video-assisted extended thymectomy in patients with thymoma by lifting the sternum. Ann Thorac Surg 2001;71:1721-3. [Crossref] [PubMed]
- Zieliński M, Czajkowski W, Gwozdz P, et al. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg 2013;44:e113-9; discussion e119.
- Detterbeck FC, Nicholson AG, Kondo K, et al. Masaoka-Koga Stage Classification for Thymic Malignancies Clarification and Definition of Terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Bieri D, Reeve R, Champion GD, et al. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation and preliminary investigation for ratio scale properties. Pain 1990;41:139-50. [Crossref] [PubMed]
- Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 1993;55:195-203. [Crossref] [PubMed]
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. [Crossref] [PubMed]
- Suda T. Single-port thymectomy using a subxiphoid approach—surgical technique. Ann Cardiothorac Surg 2016;5:56-8. [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Simorov A, Otte RS, Kopietz CM, et al. Review of surgical robotics user interface: what is the best way to control robotic surgery? Surg Endosc 2012;26:2117-25. [Crossref] [PubMed]
- Buentzel J, Straube C, Heinz J, et al. Thymectomy via open surgery or robotic video assisted thoracic surgery. Can a recommendation already be made? Medicine (Baltimore) 2017;96:e7161. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-8. [PubMed]
- Uchiyama A, Shimizu S, Murai H, et al. Infrasternal mediastinoscopic surgery for anterior mediastinal masses. Surg Endosc 2004;18:843-6. [Crossref] [PubMed]
- Numanami H, Yano M, Yamaji M, et al. Thoracoscopic Thymectomy Using a Subxiphoid Approach for Anterior Mediastinal Tumors. Ann Thorac Cardiovasc Surg 2018;24:65-72. [Crossref] [PubMed]
- Yano M, Moriyama S, Haneda H, et al. The Subxiphoid Approach Leads to Less Invasive Thoracoscopic Thymectomy Than the Lateral Approach. World J Surg 2017;41:763-70. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assistedthoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-1237.e1. [Crossref] [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [Crossref] [PubMed]
- Agasthian T. Beyond the limits, extreme minimally invasive surgery in invasive thymic tumours. J Vis Surg 2017;3:58. [Crossref] [PubMed]
- Yim AP, Kay RL, Ho JK. Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 1995;108:1440-3. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Lerut TE, et al. Thoracoscopic Thymectomy in Autoimmune Myasthenia: Results of Left-Sided Approach. Ann Thorac Surg 2000;69:1537-41. [Crossref] [PubMed]
- Zieliński M, Rybak M, Solarczyk-Bombik K, et al. Subxiphoid uniportal VATS thymectomy. J Vis Surg 2017;3:171. [Crossref] [PubMed]