
Prepectoral breast reconstruction with complete implant coverage using double-crossed acellular dermal matrixs
Introduction
In patients undergoing treatment for breast cancer, breast reconstruction can improve quality of life without negative impact on survival or cancer recurrence. Due to such benefits, the number of immediate breast reconstructions after mastectomy is steadily increasing (1). Moreover, breast reconstruction strategies are adapting to the introduction of new allogenic or xenogenic biomaterials, among which the recently developed acellular dermal matrix (ADM) has become widely used (2-5). ADMs, which can be obtained from various source materials including cadaveric, porcine, and bovine, are decellularized soft-tissue matrix grafts acting as collagen scaffolds that promote integration with the tissue by facilitating host cell repopulation and revascularization of the treated area.
ADM-based dual-plane submuscular reconstruction has become the most widely used technique for immediate breast reconstruction with silicone implant after mastectomy because this new technique partially addresses the major limitation of the classical total submuscular technique, which is the inability to recreate natural-looking ptosis due to limited lower pole expansion of the pectoralis major muscle (PMM). Nonetheless, ADM-based dual-plane submuscular reconstruction cannot overcome all limitations of the traditional technique, including persistent pain from PMM dissection and coverage, prolonged drainage, hyper-animation deformity, blunting of the natural shape due to persistent pulling of the PMM, and limited reconstruction of severely ptotic breasts (6).
To overcome these limitations, new approaches attempt to position the breast tissue together with an ADM-covered implant at the original location within the prepectoral space. Several studies have described techniques that involve inserting the silicone implant into the prepectoral space upon coverage with a pre-shaped ADM. Vidya et al. used Braxon, which is a single large ADM that can cover the entire implant, together with pre-shaped synthetic implants and reported favorable outcomes (7). Similarly, Jafferbhoy et al. and Berna et al. used Braxon to fully cover the implant according to different designs (8,9). Furthermore, Reitsamer et al. described full implant coverage using the porcine ADM Strattice (LifeCell Corporation, Bridgewater, NJ, USA) (5). Finally, Jones et al. described a technique that involved pulling an AlloDerm ADM taut over the surface of the implant (10). While these previously described techniques for prepectoral implant-based breast reconstruction differ in terms of coverage design, all require the use of a single large ADM to provide full implant coverage (11-13).
As ADMs are widely used in prepectoral implant-based breast reconstruction, we designed a technique that provides full coverage of the implant by using two smaller but adequately positioned (crossed) ADMs. Here, we summarized our experience with this new approach, providing an overview of patient selection, implant selection, and postoperative outcomes, in an effort to determine the effectiveness of the double-crossed ADM technique.
Methods
Study design
We retrospectively analyzed the records of 23 patients who underwent prepectoral breast reconstruction with silicone implant at our hospital between February 2017 and March 2018. All patients had received either skin-sparing or nipple-sparing mastectomy. Mentor or Allergan textured anatomic implants were used, together with ≥2-mm thick allogenic ADMs (CG CryoDerm; CGBio Co., Seongnam, Korea). All patients were evaluated preoperatively and followed up at 1, 3, 6, and 12 months postoperatively. The Institutional Review Board of Kyungpook National University Hospital (No. 2018-05-017-005) approved this retrospective study, and all patients provided informed consent to have their data (including de-identified photographs) recorded, analyzed, and published for research purposes.
Patient selection
We selected breast cancer patients indicated for skin- or nipple-sparing mastectomy. The other inclusion criteria were low risk of skin invasion, small-to-moderate size of the reconstructed breast, and adequate skin flap (thickness and vascularity) following mastectomy. Specifically, to minimize the risk of flap loss, we only selected patients with skin thickness ≥1.5 cm on the preoperative pinch test and with a uniform thickness of ≥0.5 cm for the subcutaneous layer in the skin flap post-mastectomy. Severely ptotic shape of the affected breast, obesity [body mass index (BMI) >30 kg/m2], diabetes mellitus, active smoking, and preoperative radiotherapy were considered exclusion criteria.
Preoperative silicone implant selection
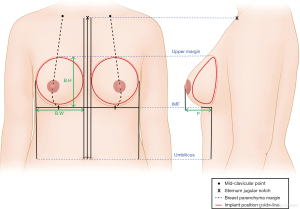
We used preoperative taping to measure the volume of both breasts. Afterwards, we conducted breast volumetric analysis (Eva® scanner; Artec 3D Inc., Luxembourg City, Luxembourg) for esthetic planning of the breast reconstruction surgery. Baseline measurements of breast width, height, projection, and skin thickness were conducted by the same operator according to well-established protocols (Figure 1). The implant size was chosen according to the breast and skin thickness measurements. Immediately after mastectomy, the most appropriate implant was selected intraoperatively using an implant sizer, to ensure breast symmetry.
Implant coverage technique using two double-crossed ADMs
The horizontal and vertical circumferences of the selected implant were measured using a measuring tape, and two appropriately sized ADMs were applied (Figure 2). The length and width of each ADM was chosen such that, when applied above and below the implant perpendicularly to each other, the ADMs would fully cover the implant. Each ADM was massively rinsed using ≥500 cm3 of betadine solution and saline in order to cleanse off the remnant cell component. Using a scalpel blade no. 11, we perforated the ADMs at intervals of 0.5–0.8 cm in the vertical and horizontal directions. Afterwards, we covered the implant with the two crossed ADMs placed with the dermal surface, which has higher probability of vasculogenesis, facing toward the tissue, not toward the implant. The margins of the ADMs were sewed with interrupted suture using 2-0 Vicryl, carefully to avoid damaging the implant (Figure 2). Cryopreserved CG Cryoderm ADMs (CGBio Co., Seongnam, Korea) were used to fully cover each implant.
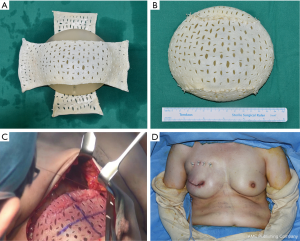
Two lines with negative suction were applied to the breast pocket (in the upper part and in the inflammatory fold), and irrigation was performed using antibiotic irrigation solutions (povidone-iodine, 50 cc; isepamicin, 80 mg; cefazolin, 1 g in 500 mL of sterile saline). After betadine re-draping, the ADM-wrapped implant was inserted into the breast pocket. Prior to insertion, we marked the midline and horizontal line of the ADM-covered implant using a marking pen to minimize implant malposition (Figure 2C). After inserting the implant into the pocket with minimal manipulation, 6–7 absorbable sutures (2-0 absorbable Vicryl) were used to bind the ADM material to the muscle tissue, from the medial part to the upper and lateral parts of the breast, in order to ensure perfect fitting and fixation of the implant into the breast pocket. In patients with a lax skin flap due to breast ptosis, absorbable sutures were applied to ensure adequate fixation of the ADM material to the subcutaneous layer and thus minimize the risk of seroma, with extra caution to not damage the anterior part of the implant. Moreover, to minimize the possibility of dead space formation in the upper pole area, 3–4 bolster sutures were performed to ensure fixation (Figure 2). Afterwards, skin closure was achieved using layer-by-layer sutures (2-0 Vicryl, 4-0 Vicryl, and 5-0 Ethilon). Negative-pressure drains are positioned anterior and posterior to the implant, and the entire breast including the incision site is covered with an external dressing (20×20 cm2) for portable negative pressure wound therapy (PICO dressing; Smith & Nephew Medical, Ltd., Hull, United Kingdom)negative-pressure wound therapy, which will help minimize the risk of seroma development due to external factors.
Data collection and analysis
We conducted chart reviews to collect data regarding baseline patient characteristics including age, BMI, history of diabetes mellitus, smoking status, preoperative breast volume, cancer pathology, cancer stage, excised mass weight, implant volume, and ADM size. Additionally, we evaluated the incidence and nature of complications, as well as patient satisfaction.
Complications were defined as major (necrosis, implant loss, capsular contracture, implant malposition/rotation, rippling, infection) or minor (dehiscence, seroma, hematoma, and red breast syndrome). Patient satisfaction surveys were conducted at 12 months after breast reconstruction, using the Kyungpook National University Hospital (KNUH) Breast Reconstruction Satisfaction Questionnaire, which uses a scale from 1 (very dissatisfied) to 5 (very satisfied).
Results
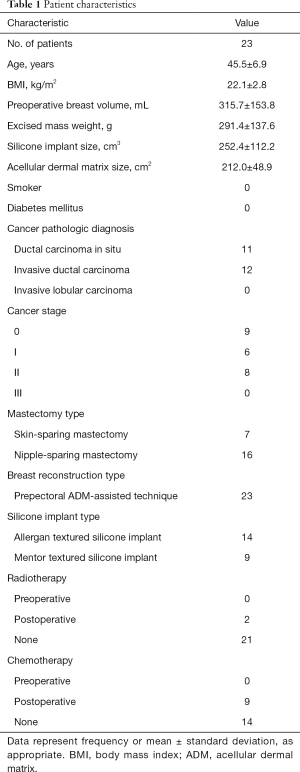
Between February 2017 and March 2018, 23 patients received prepectoral breast reconstruction using the double-crossed ADM coverage technique. The characteristics of this cohort were as follows: mean age, 45.5 years (range, 30–53 years); BMI, 22.1 kg/cm2 (range, 19.0–30.9 kg/cm2); preoperative breast volume, 315.7 cm3 (range, 150–450 cm3); excised mass weight, 291.4 g (range, 50–525 g); implant volume, 252.4 cm3 (range, 125–440 cm3). All patients were non-smokers and none had diabetes mellitus. Skin-sparing and nipple-sparing mastectomy were performed in 7 and 16 patients, respectively. Postoperative radiotherapy and chemotherapy were performed in two and nine patients, respectively, while the other patients did not receive further anti-cancer treatments (Table 1).
Full table
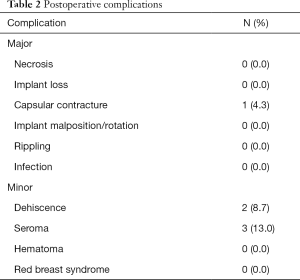
All patients received unilateral single-stage direct-to-implant breast reconstruction after mastectomy. Very few postoperative complications were noted. Only one patient experienced a major complication (capsular contracture; 1/23 patients, 4.3%) requiring implant change. Minor complications included wound dehiscence (2/23 patients, 8.7%) and seroma (3/23 patients, 13.0%) (Table 2).
Full table
At the 12-month follow-up, patient satisfaction was very high in all nine categories examined. Specifically, breast symmetry, size, shape, feel, pain, scar, self-confidence, sexual attractiveness, and overall satisfaction received scores of 4.7±0.4, 4.5±0.3, 4.7±0.5, 4.2±0.4, 4.8±0.3, 4.8±0.5, 4.6±0.4, 4.3±0.4, and 4.7±0.3, respectively (Table 3).
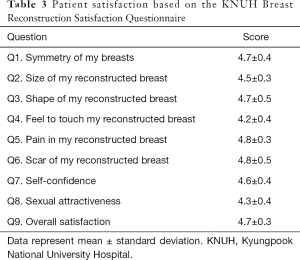
Full table
Discussion
ADM-assisted techniques for implant-based breast reconstruction are becoming increasingly popular (4). The prepectoral technique, which is an expansion of the subcutaneous technique introduced in the 1960’s, has been proposed as an alternative to the traditional dual-plane method (14). With the development of ADMs, even the dual-plane technique can produce favorable and esthetic outcomes, but limitations associated with PMM dissection and manipulation remain, including complications such as animation deformity or implant malposition. Furthermore, the dual-plane technique has a relatively large operative field and is highly invasive in nature, thus requiring intensive postoperative pain control, which often results in delayed recovery. To address such limitations, several techniques for prepectoral implant-based breast reconstruction have been reported (11). The prepectoral technique allows for positioning of the implant at the site of the excised breast tissue without damaging the PMM. By being wrapped in the ADM, the implant, which is a foreign body, can be better held in place between the muscle and skin flap (15,16).
Three main aspects should be considered when performing this prepectoral technique, namely: patient selection, implant selection, and postoperative care. Appropriate patient selection and indication are essential for the success of any surgery. Implant selection must be conducted in full consideration not only of the breast anatomy, but also of the fact that ADM coverage will be used. Thus, implant selection for ADM-assisted prepectoral reconstruction may differ slightly from the implant selection method used for dual-plane reconstruction. In addition, care should be taken so as to minimize dead space formation and seroma postoperatively, which is the most common postoperative complication; such preventive measures include ADM stitching (intraoperatively) and negative-pressure draining and dressing (postoperatively).
Here, we examined the records of 23 breast cancer patients followed up for 1 year after breast reconstruction surgery wherein two double-crossed ADMs were used to cover the breast implant, which was then inserted in the prepectoral space. We believe that the positive results obtained in our cohort can be explained in terms of the three aspects discussed above. First, we only included patients with no contraindication to prepectoral implant-based breast reconstruction (Table 1). From among the patients indicated for total mastectomy, we chose only patients indicated for skin-sparing or nipple-sparing mastectomy. Moreover, we excluded patients with conditions that might impact the skin flap negatively, including preoperative radiotherapy history, current smoker status, and uncontrollable diabetes mellitus. Furthermore, we selected only patients with skin thickness ≥1.5 cm on the preoperative skin-pinch test, as well as with adequate vascularization and uniform thickness ≥0.5 cm for the subcutaneous layer post-mastectomy. Ensuring adequate flap quality (thickness and vascularization) is necessary even if the ADM is used. Previously reported complications of prepectoral implant-based breast reconstruction, including implant visibility and rippling, can be minimized if patient selection is conducted according to the above-mentioned criteria. In addition, we excluded patients with severely ptotic breast (although this condition is rare in Asians) because, in such patients, blood supply to the post-mastectomy skin flap is generally poor and there is a high risk of implant malposition or mal-rotation within the large implant pocket during healing. Therefore, the prepectoral approach is generally recommended only in non-ptotic patients with small-to-moderate breast size.
Second, we conducted careful preoperative evaluation to ensure optimal implant selection (Figure 2). Specifically, we measured breast dimensions and breast volume (using taping), but also conducted accurate breast volumetric analysis using a three-dimensional scanner. We used the results of both analyses (taping and volumetry) to establish the breast volume and set the implant area. The implant width was initially chosen as 1–2 cm smaller than the measured breast width, with appropriate height and projection. If the patient had an adequately thick skin flap with good vascularity post-mastectomy, we conducted reconstruction using the prepectoral technique and re-assessed the optimal size of the implant in consideration of all preoperative and post-mastectomy measurements such as excised mass. Using an implant sizer, we confirmed the implant size intraoperatively prior to making the final decision regarding implant selection. When selecting the implant, it is crucial to predict the thickness of the ADM-covered implant. After choosing the implant according to this protocol, ADMs should be selected so that 2× (ADM length + ADM width) is close to but does not exceed the sum of the longest vertical and horizontal circumferences of the implant, thus ensuring full coverage of the implant. The ADMs can be bent and stretched to ensure adequate coverage. Because the ADM by itself can increase the risk of seroma, it is important to make slit incisions in both the vertical and horizontal directions to promote elongation and reduce the risk of seroma. With a slit incision, ADMs exhibit 1.3–1.5-fold increased elasticity, which facilitates using smaller ADMs for full implant coverage. This not only reduces the economic burden but provides tissue integration and granulation, which promotes healing, resulting in shorter recovery and increased patient satisfaction (Figures 3,4). We typically obtain ADM templates roughly corresponding to different implant volumes, and adjust these templates intraoperatively to wrap the selected implant. In our experience, this allows for maximum efficiency, shorter surgery duration, and improved outcomes. In addition, two of our patients received postoperative radiation therapy but did not develop any complications by the 12-month follow-up, suggesting that post-mastectomy radiation therapy is not an absolute contraindication of prepectoral implant-based breast reconstruction, in agreement with the recent findings of Elswick et al. However, larger studies with long-term follow-up are warranted to clarify this aspect (17).
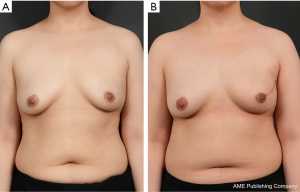
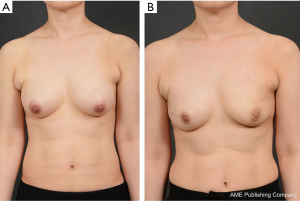
It is important to note that, after fully covering the carefully selected implant with two appropriately sized and perforated ADMs, the ADM-covered implant is directly inserted into the prepectoral plane. Since the implant is wrapped with ADMs and the medial line is not visible during this step, it is useful to mark the midline and horizontal line onto the ADM surface to assist with inserting the implant in the correct position. Afterwards, sutures can be made at different locations (from the medial part to the upper and lateral parts of the breast) in order to ensure proper fixation of the ADM-wrapped implant to the chest wall. The anterior part of the ADM-wrapped implant is also appropriately sutured to the skin flap at 2–3-cm intervals, and, finally, 3–4 bolster sutures are made near the upper pole area to minimize the risk of dead space formation. The portable negative pressure wound therapy (PICO dressing; Smith & Nephew Medical, Ltd., Hull, United Kingdom) was performed (Figure 5).
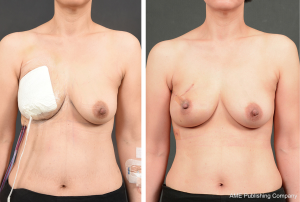
In our experience, as the surgery team becomes familiar with this technique and the protocols become established, using the prepectoral technique helps shorten and simplify the operation compared to the requirements of the traditional dual-plane technique involving the submuscular plane. This, in turn, helps reduce postoperative pain and shorten recovery. Employing various measures to prevent seroma, including adequate preoperative planning (patient selection and prepectoral implant selection), helps to further reduce recovery time and complication rates (18). Furthermore, the prepectoral technique provides aesthetically satisfactory outcomes, most likely because it allows positioning the implant in the original location of excised breast tissue (1,15,19).
In the early period after we introduced the double-crossed ADM coverage technique, one patient developed seroma which, despite persistent aspiration treatment, eventually led to capsular contracture. The patient required additional surgery using an autologous latissimus dorsi flap (Figure 6). Our experience with this particular case helped us establish a systematic treatment protocol that includes additional preoperative evaluation, selection of appropriate implant size, appropriate ADM wrapping, fixation suture of the ADM to the subcutaneous layer, and negative pressure dressing. Since then, the rate of complications (including seroma) has decreased, and no other patient required secondary surgery.
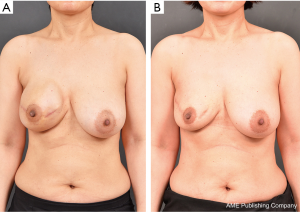
Future studies should enroll large and heterogeneous patient cohorts with long-term follow-up. Comparative analyses of the prepectoral versus traditional subpectoral plane technique should be conducted, with particular focus not only on operative outcomes but also on quality of life (including pain control and shoulder function). We are currently working in this direction.
Conclusions
Prepectoral implant-based breast reconstruction is a good alternative to traditional implant-based methods, providing numerous advantages. However, the benefits of the prepectoral technique are evident only with appropriate patient selection, implant/ADM selection, and postoperative care. Our experience suggests that, in prepectoral direct-to-implant breast reconstruction, full implant coverage using two double-crossed ADMs represents a safe and effective technique. Future studies should assess the functional and aesthetic outcomes, as well as the quality of life associated with this approach in larger and more diverse patient cohorts.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board of Kyungpook National University Hospital (No. 2018-05-017-005) approved this retrospective study, and all patients provided informed consent to have their data (including de-identified photographs) recorded, analyzed, and published for research purposes.
References
- Sbitany H. Important considerations for performing prepectoral breast reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Ibrahim AM, Koolen PG, Ashraf AA, et al. Acellular dermal matrix in reconstructive breast surgery: Survey of current practice among plastic surgeons. Plast Reconstr Surg Glob Open 2015;3:e381. [Crossref] [PubMed]
- Gandhi A, Barr L, Johnson R. Bioprosthetics: Changing the landscape for breast reconstruction? Eur J Surg Oncol 2013;39:24-5. [Crossref] [PubMed]
- Macadam SA, Lennox PA. Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery. Can J Plast Surg 2012;20:75-89. [Crossref] [PubMed]
- Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: A new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162-7. [Crossref] [PubMed]
- Vidya R, Cawthorn SJ. Muscle-sparing ADM-assisted breast reconstruction technique using complete breast implant coverage: A dual-institute UK-based experience. Breast Care (Basel) 2017;12:251-4. [Crossref] [PubMed]
- Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: First multicenter European report on 100 cases. Breast J 2017;23:670-6. [Crossref] [PubMed]
- Jafferbhoy S, Chandarana M, Houlihan M, et al. Early multicentre experience of pre-pectoral implant based immediate breast reconstruction using Braxon®. Gland Surg 2017;6:682-8. [Crossref] [PubMed]
- Berna G, Cawthorn SJ, Papaccio G, et al. Evaluation of a novel breast reconstruction technique using the Braxon® acellular dermal matrix: A new muscle-sparing breast reconstruction. ANZ J Surg 2017;87:493-8. [Crossref] [PubMed]
- Jones G, Yoo A, King V, et al. Prepectoral immediate direct-to-implant breast reconstruction with anterior AlloDerm coverage. Plast Reconstr Surg 2017;140:31S-8S. [Crossref] [PubMed]
- Vidya R, Iqbal FM. A guide to prepectoral breast reconstruction: A new dimension to implant-based breast reconstruction. Clin Breast Cancer 2017;17:266-71. [Crossref] [PubMed]
- Downs RK, Hedges K. An alternative technique for immediate direct-to-implant breast reconstruction—A case series. Plast Reconstr Surg Glob Open 2016;4:e821. [Crossref] [PubMed]
- Cattelani L, Polotto S, Arcuri MF, et al. One-step prepectoral breast reconstruction with dermal matrix-covered implant compared to submuscular implantation: Functional and cost evaluation. Clin Breast Cancer 2018;18:e703-11. [Crossref] [PubMed]
- Cronin TD, Gerow FJ. Augmentation mammaplasty: A new “natural feel” prosthesis. In: Broadbent TR. editor. Transactions of the Third International Congress of Plastic Surgery, October 13-18, 1963, Washington, DC, USA. Amsterdam: Excerpta Medica Foundation, 1964:41-9.
- Highton L, Johnson R, Kirwan C, et al. Prepectoral implant-based breast reconstruction. Plast Reconstr Surg Glob Open 2017;5:e1488. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: Rationale, indications, and preliminary results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]
- Elswick SM, Harless CA, Bishop SN, et al. Prepectoral implant-based breast reconstruction with postmastectomy radiation therapy. Plast Reconstr Surg 2018;142:1-12. [Crossref] [PubMed]
- Nahabedian MY. Current approaches to prepectoral breast reconstruction. Plast Reconstr Surg 2018;142:871-80. [Crossref] [PubMed]
- Vidya R, Green M. Minimal pain with prepectoral implant-based breast reconstruction. Plast Reconstr Surg 2019;143:236e. [Crossref] [PubMed]


