
A review of visualized preoperative imaging with a focus on surgical procedures of the breast
Introduction
Mastectomy is a preferred operative approach for the treatment of breast cancer patients in many clinical settings, and thus breast reconstruction an integral component of holistic care. The pursuit to optimize operative outcomes is particularly important given the high prevalence and rising incidence of breast cancer world-wide (1-3). Autologous breast reconstruction has been shown to be advantageous (4-6), with improved aesthetic outcomes and increased similarity to the native breast. For women with sufficient subcutaneous abdominal tissue, the anterior abdominal wall is considered the most popular donor site, and can be used for both immediate and delayed reconstructions. The muscle sparing deep inferior epigastric artery perforator (DIEP) flap has become the preferred operative approach, replacing transverse rectus abdominal (TRAM) flaps as an alternative to improve donor site morbidity (2,4,5,7).
The DIEP flap involves transfer of the abdominal subcutaneous tissue and its blood supply to the chest in order to reconstruct a new breast (4). Despite the advantages of the DIEP flap, the operation itself involves demanding microsurgical techniques, complicated by the variable anatomy of perforator location (2,7). As such, preoperative imaging has become an important part of surgical planning to ensure improved clinical outcomes. The main goal of such imaging is to inform perforator and donor site selection: the size, location, course and proximity to local structures of perforator vessels are all significant considerations. Previous research has shown optimum perforator vessels for DIEP flap breast reconstruction to be large in caliber, centrally located and of broad subcutaneous branching with limited intramuscular course (5,8).
To date, computed tomographic angiography (CTA) has been shown to be the gold standard in perforator imaging (7,9,10). This literature review evaluates CTA and the other relevant imaging modalities with a focus on their accuracy in perforator mapping and correlation with improved clinical outcomes in the context of DIEP flap surgery. Other applications for preoperative imaging in breast surgery such as imaging of alternate donor sites or of the recipient site and imaging for volumetric assessment are also discussed in brief.
Methods
A review of the literature was performed using the MEDLINE, Cochrane and PubMed databases with a focus on autologous breast reconstruction. Given the scope of imaging techniques used in preoperative imaging in surgical procedures of the breast, a number of search terms were employed. To ensure all relevant studies were included and to limit selection bias, a variety of terms for each imaging modality [ultrasound, CTA, magnetic resonance angiography (MRA), stereotaxy and three-dimensional imaging] were searched. For example, keywords ‘CTA’, ‘CT angiography’, ‘computed tomography angiography’ and ‘computed tomographic angiography’ were all included. Additional limits were applied—publication in the past 10 years and studies in English—and a manual title and abstract search was also performed.
The articles returned from the search of the literature have been used to form a qualitative analysis, with a particular focus on the outcomes of each imaging modality in preoperative imaging for surgical procedures of the breast. A description of the evolving applications of each technique has also been included.
Results
Doppler ultrasound
Doppler ultrasound is a readily accessible imaging modality with well documented use in planning free flaps. It allows for visualization of the vasculature as application of an interface layer of ultrasound gel facilitates transmission of audible signals (11). Whilst it has uses in preoperative imaging for breast surgery, Doppler ultrasound has shown to be less precise in comparison to other imaging modalities in the preoperative imaging for DIEP flap breast reconstruction (12).
A previous cohort study revealed that Doppler ultrasound was unable to locate perforator vessels, whereas imaging with CTA was accurate in locating up to five perforators per hemiabdomen (12). If suitable perforators are unable to be located, patients may be required to consent for an alternative operation (13), with the possibility of harvesting from a different donor site. Moreover, the use of preoperative Doppler ultrasound correlates in an increased surgical duration in comparison to CTA (13). This has further implications in increased intra operative blood loss and mean hospital episode, where the use of Doppler increases time spent in hospital on average by 1.3 days (13).
Despite this, there has been no significant difference in the complication rates following breast reconstruction with preoperative Doppler and CTA imaging (13,14). Moreover, there are applications for the use of Doppler in the imaging of the recipient site in autologous breast reconstruction, given the risks of radiation associated with CTA. These risks outweigh the lack of sensitivity and specificity in Doppler ultrasound imaging of the recipient site (7) and demonstrate a clinical use for Doppler in preoperative breast surgery. Doppler ultrasound is largely considered as an imaging modality that can be used as an adjunct to CTA (5).
Recent studies highlight further applications of Doppler ultrasound as a complementary imaging device to dynamic infrared thermography (DIRT). DIRT involves the detection of temperature to discern the location of vessels, and can thus be used in perforator mapping. DIRT has shown to be nearly as accurate as CTA, whilst retaining a lower risk profile with no radiation or contrast exposure (15). A prospective cohort study of 25 patients demonstrated that 95.6% of hot spots on DIRT corresponded to the accurate perforator location as determined by CTA imaging (16). However, a limitation of DIRT is the inability to locate deeper perforators: here, Doppler ultrasound can be used adjunctively as an additional non-invasive, low risk imaging modality to aid in the visualization of deeper perforators, not seen with DIRT (15).
Overall, Doppler is often used in the preoperative imaging for DIEP flap surgery and for postoperative follow-up given its ease of accessibility and reasonable accuracy in perforator mapping. Particularly, patient’s requiring imaging of the recipient site, or those receiving care in low resource settings, may benefit from preoperative imaging with Doppler ultrasound.
Color Duplex ultrasound
Color duplex ultrasound improves on Doppler technology in the addition of two-dimensional color to indicate the direction of blood flow (11). Similarly to Doppler ultrasound, this non-invasive technology also has a low risk profile with no contrast or radiation exposure (17), which may be of particular consideration in the management of patients with renal impairment, iodine allergies and those of a younger demographic and for the prevention of contrast nephropathy (2,17). Whilst color duplex is associated with a higher sensitivity and specificity in comparison to Doppler, it too is less precise than CTA in the identification of perforator vessels and is subject to much intra observer variability (5,10). In a prospective cohort study comparing color duplex to CTA, only 63% of perforators were located with color duplex in comparison to 100% with the use of CTA (17), demonstrating the low sensitivity of this technology.
Much like Doppler, color duplex ultrasound is also limited by its low accuracy with recurrent inconsistencies between imaging and operative findings (10).
MRA
Magnetic resonance imaging (MRI) involves the use of powerful magnets that cause the nuclei of hydrogen atoms to resonate, thus producing a signal that is detected by computers. With the addition of contrast in MRA, this signal can be used by computers to produce a sharper image of arteries, and thus can be applicable to the preoperative imaging of perforator flaps (11). The technology and techniques of MRA confer a number of absolute contraindications (prosthetic heart valves and implants) and relative contraindications (claustrophobia and confusion) which should be considered (2).
Several studies on the accuracy of MRA in identifying perforators in preoperative imaging suggest a high specificity (10,18), with a study on 23 patients demonstrating 100% specificity (18); however, sensitivity of MRA has been shown to be low (10,18,19) with another study on ten patients demonstrating that MRA was only able to effectively detect 91% of perforator vessels (19). In comparison, CTA has been shown to have up to 100% sensitivity, and thus continues to be the imaging modality of choice for perforators (10). Despite this, MRA, like Doppler imaging, has an important benefit in decreased radiation exposure. Non-ionizing gadolinium based contrasts (10) are popular in MRA as an alternative to radioactive contrasts, and have been shown to decrease the rate of allergic reaction by 2.93% (2). Again, MRA may be indicated in particular demographics, including patients with iodine allergies.
Non-contrast MRI also has clinical applications in breast surgery as a means of breast volume assessment, and is considered the gold standard imaging for assessment of silicone implant volume (20). For this application, MRI is considered to be more accurate than CTA and just as accurate as three-dimensional laser scanning technologies (20,21). In a study of mean calculated breast tissue using MRI and CTA, in comparison to mean actual weight resected during the operation, MRI was shown to have a Pearson correlation coefficient of >0.98, in comparison to CTA with a Pearson correlation coefficient of 0.782 (21). However, in a comparison between MRI and three-dimensional laser scanning, MRI has clear limitations due to its higher cost and decreased availability (2,20).
More recent studies have shown further applications of MRA in identifying venous anatomy prior to DIEP flap surgery as a means of reducing complications associated with postoperative venous congestion (22). Continued research and development of MRA technology may see future improvements in both accessibility and accuracy, that would promote increased clinical utility in preoperative imaging for breast surgery.
CTA
Considered the gold standard (7,9,10) in preoperative imaging for DIEP flaps, CTA is a readily available and time efficient imaging technology for the use in breast surgery (10,11). An administered bolus of venous contrast is used in CTA to produce high resolution, computer analyzed X-ray images of vascular structures (11). In the context of preoperative imaging in breast reconstruction, CTA can reconstruct the intramuscular course of both perforating vessels and other vascular structures in the field of view, in order to confirm the suitability of the donor site, as shown in Figure 1 (7,11). It is considered to be the preferred imaging modality and superior to the aforementioned techniques of Doppler imaging and MRA (7,11,24).
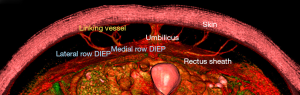
Many studies confirm the accuracy of CTA in identification of both perforator location and caliber for the use in DIEP flap preoperative planning: it has been demonstrated that the sensitivity and specificity of CTA approaches 100% (7,10,24-26). A cadaveric study found that CTA was able to locate all perforators with 96% sensitivity and perforators greater than 1 mm (considered to be clinically significant for surgical purposes) with 100% sensitivity (24). A more recent study on 106 patients demonstrated 95% concordance between perforators identified by CTA, and those selected by surgeons intraoperatively, and that this increases to 96% in patients with previously non-scarred abdomens (26). CTA has further clinical applications for the precise mapping of vasculature in other donor sites as well as in locoregional flap reconstruction, and imaging of the recipient site. However, despite these possible applications, the risks of radiation should be carefully considered when performing CTA imaging of the chest due to the proximity to remaining breast tissue, thymus and thyroid gland. Whilst there is possible utility for CTA in select situations of abnormal chest pathology or anatomy, it is suggested that a low radiation alternative such as Doppler ultrasound would be the preferred imaging modality here. This is particularly specified as it has been shown that the results from CTA imaging of recipient site vasculature are unlikely to influence the operative approach (7).
Despite the potential risk of radiation in CTA imaging of the chest, some low dose radiation protocols have been produced for CTA imaging of the donor site in DIEP flap breast reconstruction. These techniques aim to limit the field of view to the donor site on the abdominal wall without compromising the high-resolution image (11,25). One such protocol demonstrates a reduction of radiation from 8 to 6 mSv (measured using a radiation dose calculator ImPACT: CT Patient Dosimetry Calculator Version 0.99 m, ImPACT, St Georges Hospital, London, UK) which can be compared to 10 mSv from a normal computed tomography of the abdomen and pelvis as well as to the 2.4 mSv of radiation the average person is exposed to each year.
Aside from the limitations of radiation exposure, CTA has been shown to reduce the operating times, cost and complication rate in autologous breast reconstruction (11). Studies show preoperative CTA confers faster dissection and statistically significant decreased operating times (6,7,27-29) with one such study demonstrating a 54-minute decrease in operating time when compared to no preoperative imaging (6); however, a separate study (30) suggests that the reduction of operating time is only significant when considering bilateral breast reconstruction. Despite this, the same study purports the association between preoperative CTA and a statistically significant 41% reduction in mean level of operator stress (calculated using a visual analogue scale), thus demonstrating the benefit of CTA with an alternative measure. There are also trends towards decreased hospital stay; however, this data has not yet been shown to reach significance (28,29). Furthermore, the association between CTA and improved operating efficiency correlates with an overall decrease in hospital costs. This has been calculated to be between USD $3,170 (31) to $3,410 (29) per patient in two independent studies.
Several studies on complications following DIEP flap surgery also attribute decreased flap complications and donor site morbidity to preoperative CTA imaging (28,30,32). In regards to flap complications, outcomes such as fat necrosis, hematoma and seroma were measured, demonstrating greater than 45% reduction in complications in patients that received a preoperative CTA (28). A study on donor site morbidity exhibited similar trends: for exclusively bilateral cases, CTA resulted in a significant 43% reduction in complications such as herniation and bulge (30).
Similar to MRA, recent studies have also shown benefits for CTA imaging of venous anatomy prior to DIEP flap surgery. The use of identification of atypical venous connections on CTA for prediction of postoperative venous congestion proved CTA as both highly sensitive and specific (67% and 92% respectively) (33). This application of CTA holds clinical significance given venous congestion is the primary cause of flap failure following DIEP flap surgery (33).
Another discovered benefit of CTA imaging is the finding of incidentalomas such as adrenal masses, renal artery stenosis and metastatic disease (7). Whilst this is largely considered in the literature as a benefit of CTA, a study (34) has been performed to calculate the costs of the follow-up of clinically significant incidentalomas. The study shows that of the 135 patients who were investigated with CTA, 21% were discovered to have clinically significant incidental findings, thus correlating to a 32% mean increase in the cost of CTA. Importantly, concurrent CTA imaging of the chest—which only has clinical applications in select situations—increases the costs of CTA per patient 5-fold.
This review of the literature has shown routine use of CTA imaging of free abdominal wall flaps as an integral part of preoperative imaging in breast surgery. Recent developments in technology have also allowed for further applications of CTA in conjunction with software (such as Osirix: Pixmea, Geneva, Switzerland) to produce three dimensional reconstructions of the breast or area of interest (7). Improvements in MRI technology may see this modality used to equal effect alongside CTA; however, CTA currently remains as the mainstay in preoperative imaging for breast surgery across various patient groups.
Three-dimensional imaging
The use of software to produce three dimensional images from CTA is a technological advancement in the ability for spatial recognition of the anatomical location of perforators in DIEP flap surgery. As mentioned, Osirix along with other software programs such as 3D Slicer (Version 4.3, Surgical Planning Laboratory, Boston, MA, USA) have clinical applications in aiding visualization of perforator anatomy. However, the key limitation of this software is the production of these three dimensional images on a two-dimensional screen: the three-dimensional printed haptic model is a further development in the technology to counter this limitation (35). There exists a range of three-dimensional printers, including Stereolithography and Fused deposition modelling (36). These haptic models provide surgeons with tactile feedback of relevant anatomical structures, only limited by the lack of materials currently available to mimic anatomical structures.
The body of evidence for the clinical application of haptic models is with the volumetric estimation of breast tissue in breast surgery (35,37-40). This comes as a development to the more rudimentary approach of filling a bra with different specific volumes of uncooked rice to develop an estimation of breast volume (37). CTA is accurate in this volume analysis, with a study of 54 patients for DIEP flap breast reconstruction demonstrating only a 0.29% difference between CTA volumetric analysis and actual flap weight (38). Another study has also shown strong significant correlations between CTA calculations and actual volume and weight with a Pearson correlation coefficient of 0.95 and 0.97 respectively (40).
Three-dimensional imaging and printing are important preoperative tools to aid preoperative planning and both surgical trainee and patient education (35,36). An additional fourth dimension, of time, can be added to three-dimensional software to improve upon visualization of perforator vascular flow (35,36,41).
Stereotaxy
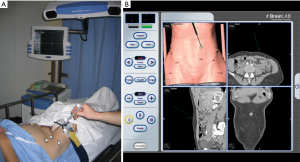
Stereotaxy involves calibration between preoperative CTA imaging and markers placed on specific anatomical landmarks with the use of an optical sensor. It allows for instantaneous feedback about the relationship between the anatomical landmark placed on the patient and the relative location of this on CTA imaging. The use of this technology is relatively new to soft tissue surgeries, as previous markers were required to be placed on bony anatomical landmarks. Recent developments in fiducial markers have enabled for the markers to be placed on the patients skin, thus enabling for this imaging technology to be applied to breast surgery (11,42). Therefore, there are limited studies available on the accuracy of this technology in preoperative breast imaging. Current evidence suggests 100% correlation between CTA and stereotaxy, with the latter producing potentially more accurate data (42,43). More research is required in the application of this imaging modality to breast surgery.
Conclusions
Preoperative imaging is integral to the planning of reconstructive breast surgery. This review has discussed the range of imaging techniques used to map and visualize perforator vasculature, and whilst there are clinical applications for the aforementioned imaging modalities, CTA has been demonstrated to be the most precise and to confer the best clinical outcomes in terms of accuracy and application. The applications of the other imaging techniques are varied and these should remain as valid alternatives, particularly for patients where radiation or contrast exposure should be limited and for low resource settings, where doppler ultrasound exists as a more accessible technology, and MRI as an evolving technique to match the objective imaging that CTA offers.
The evolution of 3D printing may improve the effective of these techniques in terms of not only donor site imaging, but recipient site imaging too, allowing planning of autologous flap design and moulding (see Figure 2) (44).

Current practices should be encouraged to evolve alongside developments in three-dimensional software and imaging, as well as stereotaxy, as improved access in the future may see these technologies become equally integral to the clinical setting. These techniques offer an adjunct to CTA and MRA in terms of perforator location (see Figure 3). Further studies could focus on the development of a more definitive protocol regarding the approach to preoperative imaging in breast surgery, as specific to different patient groups.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Chae MP, Hunter-Smith DJ, Rostek M, et al. Enhanced Preoperative Deep Inferior Epigastric Artery Perforator Flap Planning with a 3D-Printed Perforasome Template: Technique and Case Report. Plast Reconstr Surg Glob Open 2018;6:e1644. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, Rozen WM. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland Surg 2015;4:164-78. [PubMed]
- DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52-62. [Crossref] [PubMed]
- Hummelink S, Verhulst AC, Maal TJ, et al. An innovative method of planning and displaying flap volume in DIEP flap breast reconstructions. J Plast Reconstr Aesthet Surg 2017;70:871-5. [Crossref] [PubMed]
- Rozen WM, Ashton MW. Improving outcomes in autologous breast reconstruction. Aesthetic Plast Surg 2009;33:327-35. [Crossref] [PubMed]
- Wade RG, Watford J, Wormald JC, et al. Perforator mapping reduces the operative time of DIEP flap breast reconstruction: A systematic review and meta-analysis of preoperative ultrasound, computed tomography and magnetic resonance angiography. J Plast Reconstr Aesthet Surg 2018;71:468-77. [Crossref] [PubMed]
- Rozen WM, Chubb D, Grinsell D, et al. Computed tomographic angiography: clinical applications. Clin Plast Surg 2011;38:229-39. [Crossref] [PubMed]
- Rozen WM, Garcia-Tutor E, Alonso-Burgos A, et al. Planning and optimising DIEP flaps with virtual surgery: the Navarra experience. J Plast Reconstr Aesthet Surg 2010;63:289-97. [Crossref] [PubMed]
- Sommeling CE, Colebunders B, Pardon HE, et al. Lumbar artery perforators: an anatomical study based on computed tomographic angiography imaging. Acta Chir Belg 2017;117:223-6. [Crossref] [PubMed]
- Rozen WM, Stella DL, Bowden J, et al. Advances in the pre-operative planning of deep inferior epigastric artery perforator flaps: magnetic resonance angiography. Microsurgery 2009;29:119-23. [Crossref] [PubMed]
- Pratt GF, Rozen WM, Chubb D, et al. Preoperative imaging of perforator flaps in reconstructive surgery: a systematic review of the evidence for current techniques Ann Plast Surg 2013;71:246. reply. [Crossref] [PubMed]
- Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and doppler ultrasound. Plast Reconstr Surg 2008;121:1-8. [Crossref] [PubMed]
- Malhotra A, Chhaya N, Nsiah-Sarbeng P, et al. CT-guided deep inferior epigastric perforator (DIEP) flap localization -- better for the patient, the surgeon, and the hospital. Clin Radiol 2013;68:131-8. [Crossref] [PubMed]
- Klasson S, Svensson H, Malm K, et al. Preoperative CT angiography versus Doppler ultrasound mapping of abdominal perforator in DIEP breast reconstructions: A randomized prospective study. Journal of Plastic, Reconstructive & Aesthetic Surgery: JPRAS 2015;68:782-6. [Crossref] [PubMed]
- John HE, Niumsawatt V, Rozen WM, et al. Clinical applications of dynamic infrared thermography in plastic surgery: a systematic review. Gland Surg 2016;5:122-32. [PubMed]
- Weum S, Mercer JB, de Weerd L. Evaluation of dynamic infrared thermography as an alternative to CT angiography for perforator mapping in breast reconstruction: a clinical study. BMC Med Imaging 2016;16:43. [Crossref] [PubMed]
- Scott JR, Liu D, Said H, et al. Computed tomographic angiography in planning abdomen-based microsurgical breast reconstruction: a comparison with color duplex ultrasound. Plast Reconstr Surg 2010;125:446-53. [Crossref] [PubMed]
- Newman TM, Vasile J, Levine JL, et al. Perforator flap magnetic resonance angiography for reconstructive breast surgery: a review of 25 deep inferior epigastric and gluteal perforator artery flap patients. J Magn Reson Imaging 2010;31:1176-84. [Crossref] [PubMed]
- Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, et al. Preoperative planning of DIEP and SGAP flaps: preliminary experience with magnetic resonance angiography using 3-tesla equipment and blood-pool contrast medium. J Plast Reconstr Aesthet Surg 2010;63:298-304. [Crossref] [PubMed]
- Howes BH, Watson DI, Fosh B, et al. Magnetic Resonance Imaging Versus 3-Dimensional Laser Scanning for Breast Volume Assessment After Breast Reconstruction. Ann Plast Surg 2017;78:455-9. [Crossref] [PubMed]
- Kim H, Mun GH, Wiraatmadja ES, et al. Preoperative magnetic resonance imaging-based breast volumetry for immediate breast reconstruction. Aesthetic Plast Surg 2015;39:369-76. [Crossref] [PubMed]
- Dortch J, Forte AJ, Bolan C, et al. Preoperative Analysis of Venous Anatomy Before Deep Inferior Epigastric Perforator Free-Flap Breast Reconstruction Using Ferumoxytol-enhanced Magnetic Resonance Angiography. Ann Plast Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Rozen MW, Leung R, Chae MP, et al. Imaging of perforasome territories: the evolution of techniques. Aust J Plast Surg 2018;1:65-73. [Crossref]
- Rozen WM, Ashton MW, Stella DL, et al. The accuracy of computed tomographic angiography for mapping the perforators of the DIEA: a cadaveric study. Plast Reconstr Surg 2008;122:363-9. [Crossref] [PubMed]
- Rozen WM, Whitaker IS, Stella DL, et al. The radiation exposure of Computed Tomographic Angiography (CTA) in DIEP flap planning: low dose but high impact. J Plast Reconstr Aesthet Surg 2009;62:e654-5. [Crossref] [PubMed]
- Ngaage LM, Hamed R, Oni G, et al. The Role of CT Angiography in Assessing Deep Inferior Epigastric Perforator Flap Patency in Patients With Pre-existing Abdominal Scars. J Surg Res 2019;235:58-65. [Crossref] [PubMed]
- Uppal RS, Casaer B, Van Landuyt K, et al. The efficacy of preoperative mapping of perforators in reducing operative times and complications in perforator flap breast reconstruction. J Plast Reconstr Aesthet Surg 2009;62:859-64. [Crossref] [PubMed]
- Gacto-Sanchez P, Sicilia-Castro D, Gomez-Cia T, et al. Computed tomographic angiography with VirSSPA three-dimensional software for perforator navigation improves perioperative outcomes in DIEP flap breast reconstruction. Plast Reconstr Surg 2010;125:24-31. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Whitaker IS, et al. The financial implications of computed tomographic angiography in DIEP flap surgery: a cost analysis. Microsurgery 2009;29:168-9. [Crossref] [PubMed]
- Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008;28:516-23. [Crossref] [PubMed]
- Offodile AC 2nd, Chatterjee A, Vallejo S, et al. A cost-utility analysis of the use of preoperative computed tomographic angiography in abdomen-based perforator flap breast reconstruction. Plast Reconstr Surg 2015;135:662e-9e. [Crossref] [PubMed]
- Ohkuma R, Mohan R, Baltodano PA, et al. Abdominally based free flap planning in breast reconstruction with computed tomographic angiography: systematic review and meta-analysis. Plast Reconstr Surg 2014;133:483-94. [Crossref] [PubMed]
- Davis CR, Jones L, Tillett RL, et al. Predicting venous congestion before DIEP breast reconstruction by identifying atypical venous connections on preoperative CTA imaging. Microsurgery 2019;39:24-31. [Crossref] [PubMed]
- Agarwal S, Talia J, Liu PS, et al. Determining the Cost of Incidental Findings for Patients Undergoing Preoperative Planning for Abdominally Based Perforator Free Flap Breast Reconstruction with Computed Tomographic Angiography. Plast Reconstr Surg 2016;138:804e-10e. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, Rozen WM. Image-guided 3D-printing and haptic modelling in plastic surgery. In: Saba L, Rozen WM, Alonso-Burgos A, et al. editors. Imaging in plastic surgery. Taylor and Francis Press, 2014.
- Chae MP, Rozen WM, McMenamin PG, et al. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front Surg 2015;2:25. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, Spychal RT, et al. 3D volumetric analysis for planning breast reconstructive surgery. Breast Cancer Res Treat 2014;146:457-60. [Crossref] [PubMed]
- Eder M, Raith S, Jalali J, et al. Three-dimensional prediction of free-flap volume in autologous breast reconstruction by CT angiography imaging. Int J Comput Assist Radiol Surg 2014;9:541-9. [Crossref] [PubMed]
- O'Connell RL, Stevens RJ, Harris PA, et al. Review of three-dimensional (3D) surface imaging for oncoplastic, reconstructive and aesthetic breast surgery. Breast 2015;24:331-42. [Crossref] [PubMed]
- Rosson GD, Shridharani SM, Magarakis M, et al. Three-dimensional computed tomographic angiography to predict weight and volume of deep inferior epigastric artery perforator flap for breast reconstruction. Microsurgery 2011;31:510-6. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, De-Silva I, et al. Four-Dimensional (4D) Printing: A New Evolution in Computed Tomography-Guided Stereolithographic Modeling. Principles and Application. J Reconstr Microsurg 2015;31:458-63. [Crossref] [PubMed]
- Rozen WM, Buckland A, Ashton MW, et al. Image-guided, stereotactic perforator flap surgery: a prospective comparison of current techniques and review of the literature. Surg Radiol Anat 2009;31:401-8. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Stella DL, et al. Stereotactic image-guided navigation in the preoperative imaging of perforators for DIEP flap breast reconstruction. Microsurgery 2008;28:417-23. [Crossref] [PubMed]
- Chae MP, Rozen WM, Patel NG, et al. Enhancing breast projection in autologous reconstruction using the St Andrew's coning technique and 3D volumetric analysis. Gland Surg 2017;6:706-14. [Crossref] [PubMed]


