Re-visiting post-breast surgery pain syndrome: risk factors, peripheral nerve associations and clinical implications
Introduction
The introduction of the Breast Q, in 2007, as an outcome instrument for the evaluation of women having breast surgery (1) has enabled an understanding of quality of life issues in this large group of patients (2-5). Breast Q has a section addressing pain. To further evaluate pain, a numerical scoring system or the McGill pain questionnaire is typically added (6). The impact of pain in women having breast surgery is however not well defined and therefore underestimated.
In contrast, from 1986 through, and including the present (7,8), the term Post-Mastectomy Pain Syndrome has been used to discuss pain ramifications of breast interventions. The International Association for the Study of Pain (IASP) defines it as persistent pain soon after mastectomy or lumpectomy affecting the anterior thorax, axilla and/or upper arm (9). The literature related to this chronic pain after breast surgery suggests the magnitude of this problem may be as high as 50% of patients having mastectomy (10,11). However, post breast surgery pain is not limited to mastectomy patients. Other types of surgical procedures of the breast, including reconstruction after mastectomy, cosmetic breast surgery and breast reduction (12-15). Therefore, a more appropriate term to discuss pain after breast surgery that is not limited to mastectomy is “post breast surgery pain syndrome” (PBSPS) which for the purpose of simplifying our review will be used for the remainder of the manuscript.
Peripheral nerve involvement in PBSPS has been suggested by the use of topical Capsaicin to relieve pain, by directing treatment at the end terminals of the unmyelinated nerve fibers (16). In contrast, a peripheral nerve itself, the intercostal-brachial nerve, has been suggested as the cause of the pain in at least seven patients (17).
It is the purpose of this paper to revisit chronic pain as a combination of the breast intervention and relate this to the peripheral nerve(s) transmitting the pain message, in order to understand the underlying etiology of PBSPS to improve breast pain treatment outcomes.
Definition
There is no universal definition of “post breast surgery pain syndrome” (PBSPS) as it is referred to by many authors. As summarized by Waltho et al. in their literature review of 23 studies, PBSPS is pain that occurs after any breast surgery, is of at least moderate severity, possesses neuropathic qualities, is located in the ipsilateral breast/chest wall, axilla, and/or arm, lasts at least 6 months, occurs at least 50% of the time and may be exacerbated by movements of the shoulder girdle (18). Characteristics of the pain that are mostly discussed between different authors are therefore the type of pain, its severity as well as its location, duration, frequency and exacerbating factors. Duration of the pain has been argued as more than 3–6 months (10,19,20).
Jung et al. proposed a classification of neuropathic pain following breast surgery:
- Phantom breast pain (painful vs. non-painful). Non-painful is when the patient has the sensation that the removed breast tissue is still present. Painful is the same sensation but with associated pain.
- ICBN (intercostobrachial nerve) injury.
- Neuroma formation. This is the result of direct injury to nerves innervating the surgical field. Axons can be trapped in the scar formed resulting in painful neuroma formation. Ducic et al. have described danger zones of potential injury during breast surgery (21), and it is in these zones that the intercostal nerves can be injured causing the painful neuroma (15).
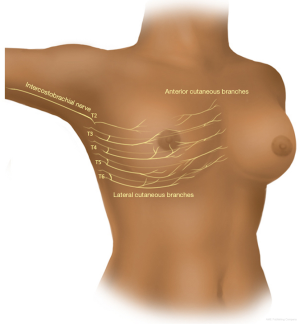
Anatomical considerations (Figure 1)
The innervation of the breast parenchyma and overlying skin is through the medial and lateral cutaneous branches of the ventral ramus of the intercostal nerves T3-T6.
The lateral cutaneous branch of the second intercostal nerve (ICBN T2) crosses the axilla to innervate the upper medial arm and the anterolateral chest wall.
The anterior branch of the lateral branch of the T2 and T3 intercostal nerves have skin territories on the anterior chest, so that numbness/pain in the axilla and upper inner arm can be combined with pain over the upper lateral breast quadrant, and cause pain in the breast with arm movements, especially overhead movement. Hence the term, “intercostobrachial” nerve (ICBN).
The nipple is innervated through the lateral branch of T4. Injury to the above will cause allodynia, paresthesias and dysesthesia, and numbness related to the PBSPS.
The pectoralis major muscle is innervated through the medial and lateral pectoralis nerves. The long thoracic nerve to the serratus runs along the posterior part of the medial wall of the axilla. The thoracodorsal nerve to the latissimus dorsi runs vertically through the axilla in proximity to the subscapular artery and vein. All the above nerves are motor, and injury to any of the above will result in paralysis or weakness of the respective muscles. These nerves have no direct cutaneous innervation. Injury to these motor nerves may give referred pain to skin territories related to the motor nerve roots for these muscles (22).
Risk factors
Risk factors associated with the development of PBSPS vary significantly between different studies. It is important to clarify that nerve injury can either be a direct traumatic nerve transection with subsequent neuroma formation, or scar tissue resulting in stretch injury. Blunt et al. discuss the potential implication of postoperative hematoma to developing pain post-mastectomy (23). Gulluoglu et al. and Amichetti et al. report post-operative radiation as potential risk factor (24,25). The mechanism underlying the radiation induced chronic pain is believed to be due to radiation neuritis, a process involving perineurial and intraneurial fibrosis, causing pain by compression of those nerve fibers, as well as the possibility loss of inhibitory input (26,27). Additionally, many authors have found young age (<40–50) to be associated with higher chances of developing the syndrome (10-12,19). Possible explanations as to why younger age is associated with higher risk are discussed by Couceiro et al. as increased nerve sensitivity and lower threshold due to anxiety, more prevalent in this age group. Also the more aggressive nature of breast cancer in the younger population and more aggressive surgical treatment in this age group result in more anxiety (19). Other authors (Couceiro et al., Wallace et al., Smith et al.) have identified high BMI (>26) as a risk factor (12,19,27).
Another risk factor identified is prior history of headaches, with the most reasonable explanation being central sensitization (28,29). Preoperative pain has been found to be a risk factor for postoperative pain after mastectomy, perceived as breast pain (phantom pain) which is used as part of the classification of the syndrome by some authors (30).
Classification
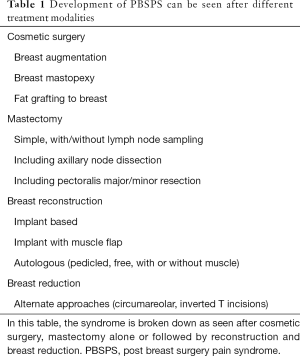
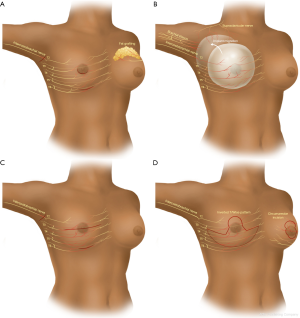
Identification of the PBSPS early in its course is paramount for prompt management and relief of what could become disabling chronic pain. We discuss different surgical approaches that can potentially result in PBSPS (Table 1, Figure 2). The type and location of surgery is also associated with direct injury to specific nerves eliciting the chronic pain (Table 2).
Full table

Full table
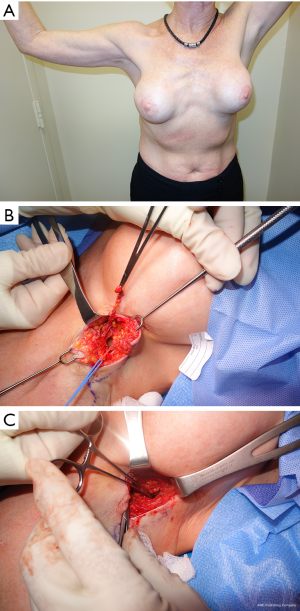
- Cosmetic breast surgery. Breast augmentation with implants can result in chronic pain in different ways. The approach/incision (peri-areolar, inframammary fold, transaxillary) can directly injure nerves or the scar tissue from the incision healing can have the same effect. Additionally, submuscular or subglandular placement of the implants and subsequent capsular contracture can cause pain from stretching adjacent structures, or entrapment of a nerve in the capsule itself. In the case of subglandular implants the skin nerve supply is involved and in case of subpectoral placement the pectoralis major may be a source of referred pain or the intercostal nerve may be adherent to the capsule around the breast implant. Fat grafting for augmentation can also result in nerve pain from direct injury during the grafting process or compression from the volume of fat injected. A clinical example is provided (Figure 3).
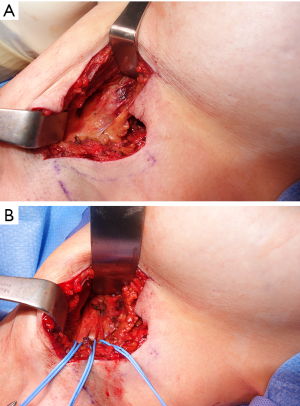
- Mastectomy or lumpectomy with or without axillary lymph node sampling or dissection. The ICBN is most commonly involved, especially during dissection in the axilla and the main reason for postoperative chronic pain following mastectomy or lumpectomy with lymph node dissection or sampling. Additionally, intercostal nerves can be injured during the process of removing the breast parenchyma. A clinical example is provided (Figure 4).
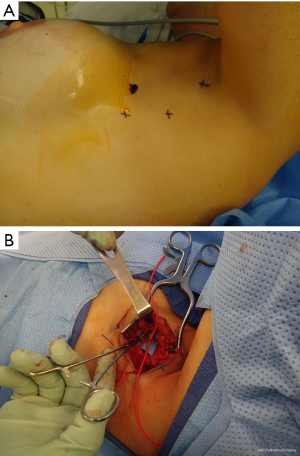
- Breast reconstruction. If the mode of reconstruction is implant-based, as discussed above, the capsular contracture and stretching of the muscle when the pocket is made sub-muscularly can cause pain. Migration of the implants can cause direct pressure to the brachial plexus in its infraclavicular location (Figure 5). Reconstruction with a pedicled or free flap that results in pain should raise concerns about direct nerve injury and neuromas during dissection of the breast pocket. A clinical example is provided (Figure 6).
- Breast reduction. Different types of incisions are used to approach the breast reduction, including an inverted-T pattern, vertical pattern and circumareolar. Regardless of the incision, the pedicle design determines the breast parenchyma to be resected. Intercostal nerves T3-T6 are invariably prone to injury depending on the pedicle design. The sensation to the nipple-areolar complex is mostly affected (decreased) with the superior pedicle.
Discussion
Persistent pain following mastectomy has been described as early as 1978 (31). However, pain can develop after any type of breast surgery and not only mastectomy. Chronic pain of this nature can be disabling and a source of considerable distress. The incidence of PBSPS is described to be anywhere between 20% and 60% (10). The resulting debilitating pain persists for years with reported incidence as high as 40% in 3 years post-breast surgery (32). This is partially due to the poor understanding of the syndrome but also the lack of appropriate treatment. Kojima et al. performed a survey among medical providers in Japan regarding identification and treatment of this syndrome (33). The authors yielded a 34.7% response rate and found that 70.5% recognized PBSPS but only 47.7% treated it. The majority prescribed NSAIDs to treat PBSPS, therefore the treatment was ineffective. This study underlines the importance of physician education in managing PBSPS. It is important to remember that PBSPS is a chronic pain syndrome and as such, it is a multifactorial condition, linked to anatomical, behavioral and socioeconomic factors (34). While the contribution of the non-anatomical factors is ambiguous, anatomical reasons for PBSPS are easier to identify and deal with. There is no universal definition and classification of the syndrome in the literature. Different terms have been used before including Post-Mastectomy Pain Syndrome (PMPS), but that term by itself only covers the cases of pain following mastectomy but no other types of breast surgery. We are therefore re-visiting the PBSPS by breaking it down to subcategories based on the location of the pain and the type of surgery. Breast augmentation, mastectomy or lumpectomy and breast reconstruction, mainly implant-based as well as breast reduction surgery can all cause pain due to different pathways of nerve injury as described above. Ducic et al. performed a systematic review to identify the incidence of chronic pain following aesthetic breast surgery and found rates between 13.57% to 15.44% (35). The nerves involved were found to be the intercostal nerves, the intercostobrachial nerve as well as the long thoracic nerve and brachial plexus (35). In the breast augmentation patients, submuscular placement of implants has been shown to result in increased pain compared to subglandular placement (50% vs. 21% respectively) (27). This is most likely related to capsular contracture and stretching of the muscle, a process that is more pronounced in the submuscular location as the nerves innervating the muscle travel underneath and potentially get incorporated within the newly formed capsule. Alternative explanation is direct brachial plexus compression, mainly from migration of the implants (13). However the systematic review by Ducic et al. showed no difference in symptoms when comparing the different implant pockets (35). The authors of this study found that implant volume is also not associated with increased risk for PBSPS (35). The intercostobrachial nerve injury is also a main risk factor for developing PBSPS after axillary lymph node dissection (ALND) or sampling for sentinel lymph node biopsy (SLNB) (10,11,17). This has led authors such as Vecht et al., who described seven cases of patients with persistent pain in the distribution of the ICBN after ALND, to suggest the use of post-axillary dissection pain syndrome to better characterize this distinct clinical entity following ALND (17). With ALND, there can be difficulty preserving the ICBN. Abdullah et al. conducted a randomized trial where patients would have their ICBN preserved during ALND or resected (36). Interestingly enough, in the nerve preservation group only in 65% was the nerve actually preserved, highlighting the difficulty in protecting the ICBN during ALND. The incidence of sensory deficits among the patients whose nerve was preserved was significantly lower at discharge and during follow-up in 3 months, highlighting the importance of nerve preservation. Implant-based breast reconstruction has found to increase the incidence of postoperative chronic pain (50% rate with implant reconstruction vs. 30% rate without) (27). The same study revealed that delayed implant reconstruction has been found to be associated with higher incidence of pain as well (59% vs. 21%) (27). An explanation of this effect is that immediate reconstruction patients are usually lower risk profile patients that can tolerate a well vascularized breast flap (either pedicled or free flap) with or without a smaller size implant and the lack of progressive tissue expansion before a larger implant is placed. Breast reduction is associated with 22% incidence of PBSPS (14). It is believed to be due to injury to intercostal and supraclavicular nerves. Injury to intercostal nerves (T2-T7) and neuroma formation as the cause of pain after either implant base reconstruction, augmentation, mastopexy or reduction has also been suggested by Broyles et al. (15). Regardless of the type of injury and the nerve involved, it is imperative that the provider involved in the care of patients complaining of pain after breast surgery is able to identify when symptoms point towards nerve injury rather than any other more common complications. Identification of the underlying etiologic factor can then tailor the appropriate treatment. This can be either surgical with nerve release and/or fat grafting or non-surgical, including pharmacologic treatments, local (capsaicin cream, botulinum toxin injections) or systemic (antidepressants) (8,35,37). The diagnostic algorithm and thorough assessment of the treatment of these nerve injuries is beyond the scope of this article.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 2009;124:345-53. [Crossref] [PubMed]
- Erdmann-Sager J, Wilkins EG, Pusic AL, et al. Complications and Patient-Reported Outcomes after Abdominal-Based Breast Reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. Plast Reconstr Surg 2018;141:271-81. [PubMed]
- Sorkin M, Qi J, Kim HM, et al. Acellular Dermal Matrix in Immediate Expander/Implant Breast Reconstruction: A Multicenter Assessment of Risks and Benefits. Plast Reconstr Surg 2017;140:1091-100. [Crossref] [PubMed]
- Mundy LR, Homa K, Klassen AF, et al. Breast Cancer and Reconstruction: Normative Data for Interpreting the BREAST-Q. Plast Reconstr Surg 2017;139:1046e-55e. [Crossref] [PubMed]
- Mundy LR, Homa K, Klassen AF, et al. Normative Data for Interpreting the BREAST-Q: Augmentation. Plast Reconstr Surg 2017;139:846-53. [Crossref] [PubMed]
- Kulkarni AR, Pusic AL, Hamill JB, et al. Factors Associated with Acute Postoperative Pain Following Breast Reconstruction. JPRAS Open 2017;11:1-13. [Crossref] [PubMed]
- Kroner K, Krebs B, Skov J, et al. Immediate and long-term phantom breast syndrome after mastectomy: incidence, clinical characteristics and relationship to pre-mastectomy breast pain. Pain 1989;36:327-34. [Crossref] [PubMed]
- Larsson IM, Ahm Sorensen J, Bille C. The Post-mastectomy Pain Syndrome-A Systematic Review of the Treatment Modalities. Breast J 2017;23:338-43. [Crossref] [PubMed]
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1-226. [PubMed]
- Couceiro TC, Valenca MM, Raposo MC, et al. Prevalence of post-mastectomy pain syndrome and associated risk factors: a cross-sectional cohort study. Pain Manag Nurs 2014;15:731-7. [Crossref] [PubMed]
- Alves Nogueira Fabro E, Bergmann A, do Amaral ESB, et al. Post-mastectomy pain syndrome: incidence and risks. Breast 2012;21:321-5. [Crossref] [PubMed]
- Smith WC, Bourne D, Squair J, et al. A retrospective cohort study of post mastectomy pain syndrome. Pain 1999;83:91-5. [Crossref] [PubMed]
- Janson RA. Implant arm: axillary compression from breast prostheses. Plast Reconstr Surg 1985;75:420-2. [Crossref] [PubMed]
- Slezak S, Dellon AL. Quantitation of sensibility in gigantomastia and alteration following reduction mammaplasty. Plast Reconstr Surg 1993;91:1265-9. [Crossref] [PubMed]
- Broyles JM, Tuffaha SH, Williams EH, et al. Pain after breast surgery: Etiology, diagnosis, and definitive management. Microsurgery 2016;36:535-8. [Crossref] [PubMed]
- Dini D, Bertelli G, Gozza A, et al. Treatment of the post-mastectomy pain syndrome with topical capsaicin. Pain 1993;54:223-6. [Crossref] [PubMed]
- Vecht CJ, Van de Brand HJ, Wajer OJ. Post-axillary dissection pain in breast cancer due to a lesion of the intercostobrachial nerve. Pain 1989;38:171-6. [Crossref] [PubMed]
- Waltho D, Rockwell G. Post-breast surgery pain syndrome: establishing a consensus for the definition of post-mastectomy pain syndrome to provide a standardized clinical and research approach - a review of the literature and discussion. Can J Surg 2016;59:342-50. [Crossref] [PubMed]
- Couceiro TC, Menezes TC, Valenca MM. Post-mastectomy pain syndrome: the magnitude of the problem. Rev Bras Anestesiol 2009;59:358-65. [Crossref] [PubMed]
- Vilholm OJ, Cold S, Rasmussen L, et al. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer 2008;99:604-10. [Crossref] [PubMed]
- Ducic I, Seiboth LA, Iorio ML. Chronic postoperative breast pain: danger zones for nerve injuries. Plast Reconstr Surg 2011;127:41-6. [Crossref] [PubMed]
- Simons David TJ, Simons L. Myofascial Pain and Dysfunction: The Trigger Point Manual. 2nd edition. Philadelphia: Lippincott Williams & Williams, 1999.
- Blunt C, Schmiedel A. Some cases of severe post-mastectomy pain syndrome may be caused by an axillary haematoma. Pain 2004;108:294-6. [Crossref] [PubMed]
- Gulluoglu BM, Cingi A, Cakir T, et al. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med 2006;51:75-82. [PubMed]
- Amichetti M, Caffo O. Pain after quadrantectomy and radiotherapy for early-stage breast cancer: incidence, characteristics and influence on quality of life. Results from a retrospective study. Oncology 2003;65:23-8. [Crossref] [PubMed]
- Basbaum AI, Gautron M, Jazat F, et al. The spectrum of fiber loss in a model of neuropathic pain in the rat: an electron microscopic study. Pain 1991;47:359-67. [Crossref] [PubMed]
- Wallace MS, Wallace AM, Lee J, et al. Pain after breast surgery: a survey of 282 women. Pain 1996;66:195-205. [Crossref] [PubMed]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis 2001;8:1-10. [Crossref] [PubMed]
- Dellon AL. Migraine and Peripheral Nerve. Pain Solutions. LaVergne: Lightning Source Press, 2013.
- Jung BF, Ahrendt GM, Oaklander AL, et al. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain 2003;104:1-13. [Crossref] [PubMed]
- Wood KM. Intercostobrachial nerve entrapment syndrome. South Med J 1978;71:662-3. [Crossref] [PubMed]
- Macdonald L, Bruce J, Scott NW, et al. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer 2005;92:225-30. [Crossref] [PubMed]
- Kojima KY, Kitahara M, Matoba M, et al. Survey on recognition of post-mastectomy pain syndrome by breast specialist physician and present status of treatment in Japan. Breast Cancer 2014;21:191-7. [Crossref] [PubMed]
- Caffo O, Amichetti M, Ferro A, et al. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat 2003;80:39-48. [Crossref] [PubMed]
- Ducic I, Zakaria HM, Felder JM 3rd, et al. Nerve Injuries in Aesthetic Breast Surgery: Systematic Review and Treatment Options. Aesthet Surg J 2014;34:841-56. [Crossref] [PubMed]
- Abdullah TI, Iddon J, Barr L, et al. Prospective randomized controlled trial of preservation of the intercostobrachial nerve during axillary node clearance for breast cancer. Br J Surg 1998;85:1443-5. [Crossref] [PubMed]
- Elia R, Nacchiero E, Vestita M, et al. "Reply to Letter to the Editor" The post-mastectomy pain syndrome-A systematic review of the treatment modalities. Breast J 2019;25:566-7. [Crossref] [PubMed]