
The potential role of carbon nanoparticles-assisted biopsy for sentinel lymph nodes of incidental thyroid carcinoma
Introduction
Thyroid nodules are a common clinical finding and the increasing prevalence of differentiated thyroid carcinoma is a challenge for thyroid surgeons since they have to identify malignant nodules, which represent 5–15% of thyroid nodules. Although the majority of malignant nodules can be diagnosed by fine needle biopsy (FNA) and ultrasound examination preoperatively, incidental thyroid carcinoma (i.e., malignant finding of a nodule that was initially thought as benign) identified in specimens of thyroidectomy performed for benign nodules is quite frequent (1). Thyroid microcarcinoma represents most of the incidental carcinoma since they are difficult to be sampled adequately by FNA. Hence, the diagnosis of differentiated thyroid cancer may be missed. The majority of follicular variants of thyroid papillary carcinoma are encapsulated and the neoplastic cells do not show the nuclear features of papillary carcinoma. As a result, the diagnoses of these tumors may be missed even by experienced pathologists (2).
In this situation, the presence of lymph nodes metastasis provides additional information and may help diagnose thyroid carcinoma intraoperatively. As reported, papillary thyroid carcinoma involves cervical lymph nodes in 20–50% of the cases (3). The spread of tumors usually begins in the perithyroidal lymph nodes and progresses to the lateral neck compartments (4,5). Given that ‘skip’ metastases occur rarely, especially when the primary tumor located in upper thyroid pole, perithyroidal lymph nodes are considered to be the first metastases station, i.e., sentinel lymph node (SLN) (6). Nevertheless, preoperative investigation of perithyroidal lymph nodes is technically difficult and unreliable by ultrasound and only half of lymph nodes metastasis in central neck compartment can be observed by the naked eye during the operation (7).
Ito et al. (8) suggest that for small incidental thyroid cancer <1 cm, observation is appropriate in the absence of unfavorable features. Nevertheless, incidental carcinoma may behave aggressively in some cases, particularly in those with unfavorable histopathological features (9). Nevertheless, some cancers are found in the surgical specimens after thyroidectomy for benign lesions, and the correct management of these patients is still unknown. This finding not only inflicts additional suffering and financial burden on the patients, but also implies the possible need for further treatments and surgeries. Kr et al. (10) suggested that incidental lymph node metastasis from thyroid cancer found during head and neck should prompt the search for the primary lesion.
Therefore, there is a need to find effective solutions to prevent thyroid microcarcinoma from being preoperatively and intraoperatively missed. Carbon nanoparticles (CNs) suspension has been widely used to assist to clean the lymph nodes by dyeing them during breast, gastric and thyroid surgery (11,12). Also it has been used to protect the function of parathyroid glands. We began in February 2012 to explore the differential and protective effects of CNs on parathyroid gland in thyroid surgery. With this objective in view, we use CNs as a lymph node tracer to perform SLN biopsy (SLNB) during thyroid surgery to increase the possibility of detecting small aggressive thyroid cancers.
Methods
Study design and patients
This was a retrospective study of 541 consecutive patients who underwent thyroid surgery for benign thyroid tumors that were later determined as thyroid cancer, either during or after surgery. The surgeries were performed between February 2012 and February 2014 at the Thyroid Department of the Hunan Provincial People’s Hospital. This study was approved by the Medical Ethics Committee of Hunan Provincial People’s Hospital, Changsha, China (Ethic Number: 201612010). The need for individual consent was waived by the committee because of the retrospective nature of the study.
All patients were diagnosed with thyroid benign lesions both preoperatively by FNA and intraoperatively by frozen biopsy. The surgical indications for the benign tumors were (13): (I) presence of localized oppression symptoms that were significantly associated with nodules; (II) hyperthyroidism, which could not be alleviated with medical treatment; (III) tumor located behind the sternum or in the mediastinum; or (IV) the nodules were growing and could be considered as having a malignant tendency or were complicated with risk factors for thyroid cancer. Among the 541 patients, 392 underwent intraoperative SLNB while the other 149 did not. All tumor diagnoses were confirmed by two pathologists (deputy chief physician or above) using standard diagnostic criteria on pathological section (14). The nodules that were found incidentally during imaging examination were considered as incidental thyroid nodules (1). The incidental thyroid cancers were diagnosed according to the guidelines (15).
Surgical procedures
All surgeons were in the same team, and all of them were associate chief physician or above. General anesthesia was given to all patients. The patients were placed in the supine position. A cervical collar curved incision was made in the anterior cervical region to expose the prethyroid region including the paratracheal region and the isthmus of the thyroid gland. The injection of CNs in the central compartment lymph node was performed as previously described (16). At the previously chosen one to four sites on the thyroid surface, 0.1–0.3 mL of CNs was injected into the superficial thyroid gland 2–3 mm in depth. Prior to being injected, negative pressure was applied before administration to make sure there was no intravenous CNs injection. The flow of CNs inside the thyroid tissue and lymphatic vessels was observed. After 1–5 min, the lymph nodes stained black by CNs began to be sighted in the pretracheal region. We defined the first blacked lymph nodes as SLNs, including those in the prelaryngeal region, the region below the isthmus and on the tracheal surface, and the regions adjacent to the upper and lower poles of the thyroid, or those in the regions adjacent to the recurrent laryngeal nerves and/or in other parts of the central compartment. The first black stained SLNs were removed and submitted for frozen section analysis (as shown in Figure 1). Meanwhile, thyroid surgery was performed and relevant specimens were submitted for frozen section analysis.
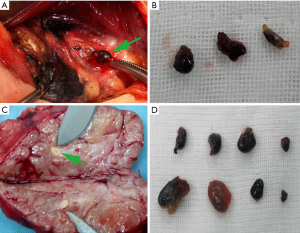
Statistical analysis
Only descriptive statistics were presented. Continuous variables were presented as mean ± standard deviation. Categorical data were presented as frequencies.
Results
Characteristics of the patients
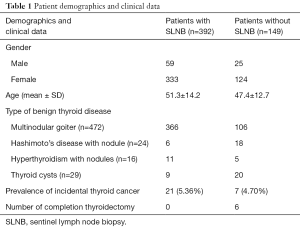
The demographic data are presented in Table 1. The patients’ age ranged from 15 to 78 (mean, 39).
Full table
Preoperative diagnoses
As shown in Table 1, the preoperative diagnoses of the 541 patients were nodular goiter (n=472), Hashimoto’s disease with nodules (n=24), hyperthyroidism with nodules (n=16), and thyroid cysts with obstructive symptoms (n=29).
SLN biopsy (SLNB)
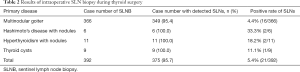
Of 392 SLN biopsies conducted during operation, SLNs could be sampled in 375 cases (95.7%). SLNs failed to be detected in 17 patients who only presented with thyroid nodules. Table 2 presents the rates of SLNB according to the preoperative diagnosis.
Full table
Among the 375 successful biopsies, SLN metastasis of thyroid microcarcinoma was confirmed in 21 cases without intraoperatively detected thyroid microcarcinoma in the corresponding thyroid tissue and/or tumor/nodules by frozen section biopsy. All incidental nodes with thyroid carcinoma were microcarcinoma. The mean tumor size is 5.6±2.4 mm. Accordingly, these 21 patients received radical surgical treatment for thyroid carcinoma during the initial operation and avoided undergoing a second one (Table 2).
Of the 149 patients who did not have intraoperative SLNB, seven were diagnosed with thyroid microcarcinoma by postoperative paraffin section analysis of thyroid nodules. The rate of incidental thyroid carcinoma was 4.7% (7/149, the mean tumor size is 5.9±2.2 mm). One patient out of those seven had a near-total thyroidectomy during the first surgery and thus avoided undergoing a second surgery. The remaining six patients, however, had to undergo a second surgery later.
Discussion
Some thyroid cancers are found after thyroidectomy for benign lesions, implying additional surgery and treatments. There is a need to find effective solutions to prevent thyroid microcarcinoma from being preoperatively and intraoperatively missed. Therefore, the present study aimed to investigate the role of intraoperative SLNB for diagnosis of incidental thyroid carcinoma. The results suggest that intraoperative SLNB could help diagnose differentiated thyroid microcarcinoma that may be missed both preoperatively and intraoperatively. This approach could prevent the patients from having to undergo a second surgery since the intraoperative frozen section examination of the SLNs can reveal metastasis from thyroid cancer.
According to reported literature, incidental thyroid carcinoma has an incidence of about 5–15% in patients treated operatively for benign thyroid diseases (1,17,18) and the diagnosis of differentiated thyroid cancer may be missed, even by experienced pathologists (2). In this situation, the presence of lymph nodes metastasis provides additional information and may help diagnose thyroid carcinoma intraoperatively (3). Preoperative investigation of perithyroidal lymph nodes is technically difficult and unreliable by ultrasound and only half of lymph nodes metastasis in central neck compartment can be observed by the naked eye during the operation (7). Therefore, SLNB could be an appropriate approach and can be performed with low-risk techniques such as CNs.
As reported in the literature, 18–87% of those missed well-differentiated thyroid carcinomas were diagnosed as multifocal thyroid carcinoma by postsurgical pathologic analyses, and the ratio reported by Li et al. (19) and Park et al. (20) was around 37% (20). According to the NCCN guidelines, incidentally discovered papillary thyroid carcinomas of 1–4 cm in size may warrant a completion thyroidectomy for lymphovascular invasion, although observation is another option for some patients (21). In addition, ATA guidelines suggest that completion thyroidectomy may be required when the diagnosis of malignancy is confirmed following lobectomy to ensure of complete resection of multicentric disease and allow for efficient radioactive iodine therapy (22). Nevertheless, reoperation not only inflicts additional pain and financial burden on these patients but also challenge surgeons for increased risk of possible complications. Thus, the key to avoid the aforesaid unfavorable situations and consequences brought by a second surgery is to effectively prevent missing diagnosis of thyroid carcinoma prior to or during the initial thyroid surgery. In the present study, SLNs were detected in 375 (95.7%) of the 392 with intraoperative SLNB and 21 were confirmed to be with lymph node metastasis of thyroid carcinoma. On the other hand, seven out of 149 patients (4.7%) who did not receive intraoperative SLNB were diagnosed with thyroid cancer by postoperative paraffin section analysis. All incidental nodes with thyroid carcinoma were microcarcinoma. This may be related to the uncertainty of Thyroid Imaging, Reporting and Data System (TIRADS) and FNA results, and the uncertainty of these methods is mainly concentrated in microcarcinoma evaluation (23,24). Completion surgeries had to be performed in six patients due to insufficient extent of initial surgical operation.
These results are important in terms of patient management. Indeed, finding a positive SLN intraoperatively not only leads to total thyroidectomy and prevents a second procedure, but it is also an ideal surgical treatment during the initial surgery, which is therapeutic central neck dissection and leading to accurate staging and risk stratification of these patients. This is just as important if not more significant than preventing a second procedure. Finding metastatic disease may lead to radioactive iodine treatment instead of total thyroidectomy alone, potentially decreasing recurrence. Such cancers were detected at rates of 5.6% and 4.7% in the excised thyroids from patients with and without a SLNB, respectively. That is in fact lower than some reported estimates (1,9,16-18). The fact is, there is no consensus about treatment of microcancers and consider that if not for the benign lesion indicating surgery, none of these patients would have been discovered and the longer-term clinical course of these cancers is unknown. Some of them could have progressed rapidly, but it is also probable that many of them were relatively indolent disease that progressed over many years. Additional studies will have to be performed to determine the long-term natural course of this disease. Nevertheless, Ito et al. (8) suggests that small thyroid cancers <1 cm could be simply observed. This point warrants further investigation.
SLNB is a routine procedure in cancers such as breast cancer (25). Moreover, occult breast cancers may be diagnosed through the sampling of suspicious SLNs in the axilla (26,27). In light of these facts and inspired by our previous successful experience in using CNs as lymph node tracer in differentiated thyroid carcinoma requiring reoperation, we investigated potential role of CNs-assisted SLNB in the diagnosis of incidental thyroid carcinoma intraoperatively (16). Numerous studies have demonstrated the role of SLNB in cervical neck dissection using patent blue and radiotracers with dynamic lymphoscintigraphy (28,29). CNs have recently been used to identify lymph nodes and protect parathyroid gland during thyroid cancer surgeries (19) and cervix cancer (30). NC suspension, with a particle diameter of 150 nm and a high tropism for the lymphatic system, can quickly enter the lymphatic circulation after being uptaken by macrophages and without entering the blood vessels when injected into the thyroid tissue (31). As the CNs gather and deposit in the lymph nodes, the thyroid lymph drainage area will be dyed black and the perithyroidal lymph nodes will be their first stop (32).
In the present study, 392 SLN biopsies were performed to detect 21 microcancers and prevent a second operation compared to six patients requiring reoperation for microcancers. It is extremely likely that lymph node metastases occur in patients with macrocancers, but lymph node biopsy is not a routine practice in macrocancers and we do not know the significance of lymph node metastases in well-differentiated thyroid carcinoma (33-35). Sampling lymph nodes in well-differentiated thyroid carcinoma is controversial and could be beneficial only in selected patients (33,34,36,37).
As SLNB in thyroid cancer management is still under study at the present time, no criteria have been established for SLNs biopsy for thyroid carcinoma. Although application of costly CNs undoubtedly increases the healthcare costs, the identification rate of SLNs was up to 95.7% and 21 patients may have derived benefits from it. Studies suggested that SLNB may help guide neck dissection, patient surveillance, and radioactive iodine therapy in thyroid carcinoma (6,38).
The present study is not without limitations. The sample size was small and from a single center. Because of the retrospective nature of the study, we could not perform predictive analyses of the presence of positive SLNs based on the clinical features of the patients. Finally, follow-up data were unavailable for many patients. Additional studies are necessary to overcome these issues.
In conclusion, we should bear in mind that patients with benign thyroid tumors have a certain combination rate of malignant nodes. Intraoperative SLNB could help diagnose differentiated thyroid microcarcinoma that may be missed both preoperatively and intraoperatively. This approach may prevent patients from having to undergo a second surgery since the intraoperative frozen section examination of the SLNs can reveal metastasis from thyroid cancer. However, the indication of specific conditions rather than routine use of CNs in surgery of benign thyroid tumors has yet to be studied.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of Hunan Provincial People’s Hospital, Changsha, China (Ethic Number: 201612010). The need for individual consent was waived by the committee because of the retrospective nature of the study.
References
- Bradly DP, Reddy V, Prinz RA, et al. Incidental papillary carcinoma in patients treated surgically for benign thyroid diseases. Surgery 2009;146:1099-104. [Crossref] [PubMed]
- Albores-Saavedra J, Wu J. The many faces and mimics of papillary thyroid carcinoma. Endocr Pathol 2006;17:1-18. [Crossref] [PubMed]
- Hughes DT, Doherty GM. Central neck dissection for papillary thyroid cancer. Cancer Control 2011;18:83-8. [Crossref] [PubMed]
- Sturgeon C, Yang A, Elaraj D. Surgical Management of Lymph Node Compartments in Papillary Thyroid Cancer. Surg Oncol Clin N Am 2016;25:17-40. [Crossref] [PubMed]
- Lee YS, Shin SC, Lim YS, et al. Tumor location-dependent skip lateral cervical lymph node metastasis in papillary thyroid cancer. Head Neck 2014;36:887-91. [Crossref] [PubMed]
- Cabrera RN, Chone CT, Zantut-Wittmann D, et al. Value of sentinel lymph node biopsy in papillary thyroid cancer: initial results of a prospective trial. Eur Arch Otorhinolaryngol 2015;272:971-9. [Crossref] [PubMed]
- Kim KE, Kim EK, Yoon JH, et al. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg 2013;37:385-91. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010;34:28-35. [Crossref] [PubMed]
- Ardito G, Revelli L, Giustozzi E, et al. Aggressive papillary thyroid microcarcinoma: prognostic factors and therapeutic strategy. Clin Nucl Med 2013;38:25-8. [Crossref] [PubMed]
- Kr A, Sebastian P, Somanathan T, et al. Significance of incidentally detected thyroid tissue in lymph nodes of neck dissections in patients with head and neck carcinoma. Int J Surg Pathol 2012;20:564-9. [Crossref] [PubMed]
- Zhang D, Wang T, Dionigi G, et al. Application of Carbon Nanoparticles in Endoscopic Thyroidectomy via Bilateral Areola Approach: Total Thyroidectomy Plus Central Lymph Node Dissection. J Laparoendosc Adv Surg Tech A 2019;29:1038-41. [Crossref] [PubMed]
- Wang B, Su AP, Xing TF, et al. The function of carbon nanoparticles to improve lymph node dissection and identification of parathyroid glands during thyroid reoperation for carcinoma. Medicine (Baltimore) 2018;97:e11778. [Crossref] [PubMed]
- Endocrinology Branch of Chinese Medical Association, Surgery Branch of Chinese Medical Association, China Anti-Cancer Association Head and Neck Tumor Professional Committee, et al. Guidelines for the diagnosis and treatment of thyroid nodules and differentiated thyroid carcinomas. Chin J Clin Oncol 2012;39:1249-72.
- Bychkov A. Thyroid gland - Thyroid cancer - World Health Organization (WHO) classification. In: Wenig BM. editor. Atlas of Head and Neck Pathology. Philadelphia: Elsevier, 2018.
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Chaojie Z, Shanshan L, Zhigong Z, et al. Evaluation of the clinical value of carbon nanoparticles as lymph node tracer in differentiated thyroid carcinoma requiring reoperation. Int J Clin Oncol 2016;21:68-74. [Crossref] [PubMed]
- Sakorafas GH, Stafyla V, Kolettis T, et al. Microscopic papillary thyroid cancer as an incidental finding in patients treated surgically for presumably benign thyroid disease. J Postgrad Med 2007;53:23-6. [Crossref] [PubMed]
- Miccoli P, Minuto MN, Galleri D, et al. Incidental thyroid carcinoma in a large series of consecutive patients operated on for benign thyroid disease. ANZ J Surg 2006;76:123-6. [Crossref] [PubMed]
- Li Y, Jian WH, Guo ZM, et al. A Meta-analysis of Carbon Nanoparticles for Identifying Lymph Nodes and Protecting Parathyroid Glands during Surgery. Otolaryngol Head Neck Surg 2015;152:1007-16. [Crossref] [PubMed]
- Park SY, Park YJ, Lee YJ, et al. Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer 2006;107:1831-8. [Crossref] [PubMed]
- Tuttle RM, Haddad RI, Ball DW, et al. Thyroid carcinoma, version 2.2014. J Natl Compr Canc Netw 2014;12:1671-80; quiz 80.
- Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2017;27:481-3. [Crossref] [PubMed]
- Chen L, Chen L, Liu J, et al. The Association Among Quantitative Contrast-Enhanced Ultrasonography Features, Thyroid Imaging Reporting and Data System and BRAF V600E Mutation Status in Patients With Papillary Thyroid Microcarcinoma. Ultrasound Q 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Partyka KL, Wu HH. Fine-needle aspirates of thyroid microcarcinoma. J Am Soc Cytopathol 2017;6:236-41. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights Breast Cancer, Version 1.2016. J Natl Compr Canc Netw 2015;13:1475-85. [Crossref] [PubMed]
- Sohn G, Son BH, Lee SJ, et al. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: a nationwide retrospective study. J Surg Oncol 2014;110:270-4. [Crossref] [PubMed]
- Gusic LH, Johnson R. Occult Primary Breast Cancer with Axillary Metastases. In: Aydiner A, Igci A, Soran A. editors. Breast Disease: Management and Therapies. Geneva: Springer International Publishing Switzerland, 2016.
- Boschin IM, Pelizzo MR, Giammarile F, et al. Lymphoscintigraphy in Differentiated Thyroid Cancer. Clin Nucl Med 2015;40:e343-50. [Crossref] [PubMed]
- Pelizzo MR, Merante Boschin I, Toniato A, et al. Sentinel node mapping and biopsy in thyroid cancer: a surgical perspective. Biomed Pharmacother 2006;60:405-8. [Crossref] [PubMed]
- Lu Y, Wei JY, Yao DS, et al. Application of carbon nanoparticles in laparoscopic sentinel lymph node detection in patients with early-stage cervical cancer. PLoS One 2017;12:e0183834. [Crossref] [PubMed]
- Yang F, Jin C, Yang D, et al. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur J Cancer 2011;47:1873-82. [Crossref] [PubMed]
- Hagiwara A, Takahashi T, Sawai K, et al. Lymph nodal vital staining with newer carbon particle suspensions compared with India ink: experimental and clinical observations. Lymphology 1992;25:84-9. [PubMed]
- Wang TS, Dubner S, Sznyter LA, et al. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch Otolaryngol Head Neck Surg 2004;130:110-3. [Crossref] [PubMed]
- Paschke R, Lincke T, Muller SP, et al. The Treatment of Well-Differentiated Thyroid Carcinoma. Dtsch Arztebl Int 2015;112:452-8. [PubMed]
- Podnos YD, Smith D, Wagman LD, et al. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg 2005;71:731-4. [PubMed]
- Noguchi M, Kumaki T, Taniya T, et al. Bilateral cervical lymph node metastases in well-differentiated thyroid cancer. Arch Surg 1990;125:804-6. [Crossref] [PubMed]
- Spriano G, Ruscito P, Pellini R, et al. Pattern of regional metastases and prognostic factors in differentiated thyroid carcinoma. Acta Otorhinolaryngol Ital 2009;29:312-6. [PubMed]
- Jozaghi Y, Richardson K, Anand S, et al. Frozen section analysis and sentinel lymph node biopsy in well differentiated thyroid cancer. J Otolaryngol Head Neck Surg 2013;42:48. [Crossref] [PubMed]


