
Surgical management of adrenal tumours extending into the right atrium
Introduction
Adrenal gland tumours are relatively common, affecting 3% to 10% of the population (1). Small benign non-functional adrenocortical adenomas account for the majority of these tumours. Adrenocortical carcinomas, however, are rare, with an incidence of 1 to 2 per million cases per year. One out of three patients with adrenocortical carcinoma presents with involvement of the venous system and inferior vena cava (IVC) thrombus (2). Tumours of the right adrenal gland are more likely to involve the IVC, due to the right adrenal vein directly draining into it (3).
The aim of this report is to present our surgical experience of using cardio-pulmonary bypass (CPB) associated with deep hypothermic circulatory arrest (DHCA) for the treatment of adrenal tumours extending into the right atrium (RA).
Classification of tumours/thrombus for adrenal tumour
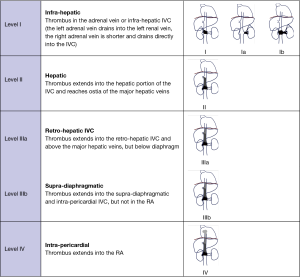
A classification in four levels has been described according to the location of the upper limit of the tumour/thrombus (Table 1). The technique used for intra-operatory venous control depends on the location and extension of the tumour/thrombus in the venous circulation (2,4,5).

Full table
Surgical techniques for venous control
Three different techniques can be used according to the level of extension of the tumour/thrombus in the venous circulation (6).
- Cross clamping of the IVC is sufficient if the upper limit of the tumour/thrombus is below the hepatic veins (level I).
- Hepatic vascular exclusion (HVE) is the technique of choice for tumour/thrombus extending into the hepatic veins or into the retro- or supra-hepatic IVC, but below the diaphragm (level II and IIIa). HVE is generally well tolerated, provided there is adequate fluid expansion before clamping.
- Treatment of tumours/thrombus extending into the cavoatrial junction or the RA (level IIIb and IV) requires the use of concomitant CPB. The advantage of this approach is that it provides haemodynamic stability during cross clamping of the IVC, reduces the risk of cardiac arrest and facilitates surgical dissection. Our technique of choice is the association of CPB with hypothermic circulatory arrest (HCA). This approach provides a bloodless operating field and direct intra-vascular vision, reduces the risk of embolization and allows extensive IVC or RA repair in cases of infiltration of the vascular wall.
A summary of the different surgical approaches is reported in Figure 1.
Surgical planning and CPB + HCA technique
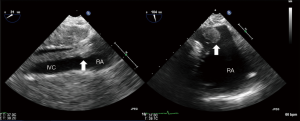
Patients who present with an adrenal mass extending into the supra-diaphragmatic IVC are routinely discussed with the cardiac surgical team in a multidisciplinary team (MDT) setting. Pre-operative assessment includes CT scan and trans-thoracic echocardiography to assess the extension of the tumour and plan the surgical strategy (Figure 2).
The procedure is performed in the cardiac theatre, under the care of a wide team composed by a general/endocrine surgeon, a cardiac surgeon, a cardiac anaesthetist, a trans-oesophageal echocardiography (TOE) operator, a perfusionist and a cardiac scrub nurse. Taking into account that these are long procedures, we usually scheduled them as all-day cases.
TOE assessment plays an important role in planning the surgical technique, guiding the venous cannulation and ensuring that the mass is totally removed.
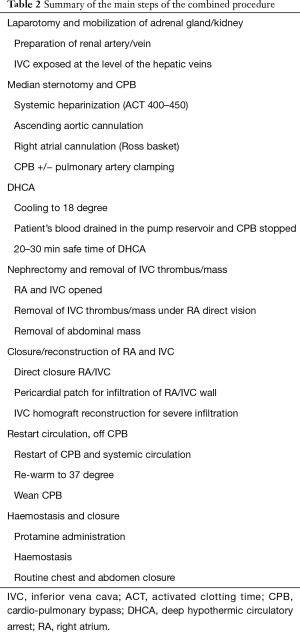
The surgical procedure starts with a laparotomy and the general surgeon mobilising the adrenal gland, the kidney and gaining control of the infra-diaphragmatic IVC. Once the dissection in the abdomen is completed, the cardiac surgeon proceeds with a median sternotomy, opening of the pericardium and systemic heparinization. It is important to maximise the amount of abdominal dissection performed before heparin administration to minimize the bleeding. Two Tycron 3.0 pursing sutures are placed at the level of the ascending aorta; a 24F aortic cannula is inserted and connected to the arterial line. A single Tycron 3.0 pursing is placed on the RA. A large atrial incision is performed to facilitate the introduction of a Ross basket (Figure 3) and establish the venous drainage. The rational of using a Ross basket instead of an ordinary venous cannula is to minimize the risk of dislodgement of the atrial mass. The venous cannulation is performed under TOE guidance to minimize the risk of embolization of the atrial mass. A small cannula is inserted into the ascending aorta, proximally to the arterial cannulation site for the cardioplegia administration.
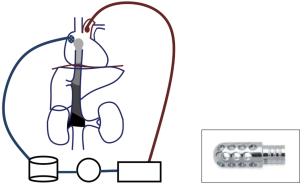
A schematic view of CPB is described in Figure 3 and a summary of the main steps of the procedure in Table 2. CPB is established and the patient is cooled to 18 degrees. This process takes 20–30 minutes, depending on the body surface area of the patient. As response to hypothermia the heart fibrillates and a vent is placed into the right superior pulmonary vein to avoid left ventricular distension. Once the cooling is completed a cross clamp is applied to the ascending aorta and a cardioplegic solution is administrated into the aortic root. A clamp can be applied across the pulmonary artery to reduce the risk of pulmonary embolisation.
Full table
The circulation is arrested, the venous blood is drained into the pump reservoir and the CPB is stopped. The DHCA provides 20–30 minutes of safe bloodless field for both surgeons to expose the IVC and the RA, remove the thrombus and reconstruct the structures.
During DHCA the cerebral perfusion is interrupted, the low temperature protects the brain, but the risk of ischemic injury increases exponentially after 35–40 minutes (7,8).
During the DHCA both surgeons operate simultaneously. A right atriotomy and IVC cavotomy are performed to expose both abdominal and intrapericardial mass. The cardiac surgeon then carefully mobilised the mass, free from the wall of the IVC and RA.
The tumour/thrombus is pulled down into the IVC and pushed down from the atrium by both surgeons. The IVC and RA are carefully inspected to ensure that no thrombus is left behind. The IVC and the RA are directly sutured with 4.0 Prolene. For infiltrating tumours a bovine or autologous pericardial patch can be used to reconstruct or augment the IVC or the RA wall. In one case we used a homograft to replace an entire segment of the IVC, which was severely infiltrated by the tumour.
Once the structures are reconstructed, the circulation and the CPB are restarted and a rigorous de-airing drill is performed. The blood is re-warmed to 37 degrees. During this period the patient is coagulopathic due to the low temperature, the inflammatory response to CPB and the systemic heparinization.
Once a physiological temperature has been reached and maintained for few minutes, the patient is weaned and disconnected from CBP. TOE is used to assess the heart function, the right atrial cavity, tricuspid function and ensure that there is no significant gradient/obstruction across the IVC in the cases requiring a surgical reconstruction. Protamine reversal is administrated. A systematic haemostasis is performed before proceeding with the closure of the chest and the abdomen. Clotting factors and platelets transfusion may be required to optimise coagulation status and haemostasis.
The Oxford experience
Due to the low incidence of ACC, the personal surgical experience for locally advanced tumours remains limited (9). In our unit the annual workload over the last decade has been in excess of 70 cases/year. Out of this large cohort of patients, nine patients with infra-hepatic IVC tumours extension were operated without establishing CPB (Figure 4). Cardiopulmonary bypass was used in seven patients with tumour/thrombus extending in the supra-diaphragmatic IVC (Figure 5) and DHCA in only two patients.
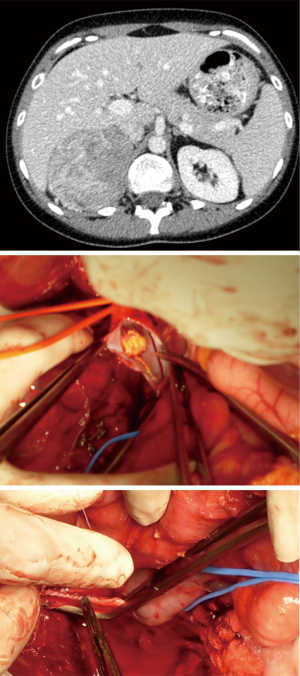
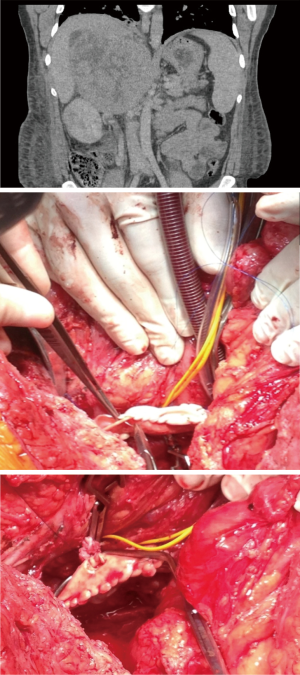
Out of the 7 patients treated in Oxford with tumour/thrombus extending in the supra-diaphragmatic IV requiring cardiopulmonary bypass some patients experienced unexpected long disease-free survival. A patient operated in 2007 remains disease free (32 years, Cushing syndrome, left adrenal tumour with thrombus into the atrium) and patient described in Figure 4 is disease-free over 4 years after the operation. There was a single in-hospital death (day 11 postop due to hypoxic brain injury and multiorgan failure).
The most recent case needing HCA was a 61-year-old man with a 12-cm malignant right phaeochromocytoma extending into the RA (Figure 6). After a 4-week adrenergic blockade he underwent right radical adrenalectomy. The HCA time was 14 minutes. His post-operative recovery was uneventful and he was discharged home on post-operative day 10. Histology showed an adrenal phaeochromocytoma with PASS score of 15/20. At 4 weeks after the operation, biochemistry was normal (metanephrine 0.25 µmol/24 h, normetanephrine 2.31 µmol/24 h). An metaiodobenzylguanidine (MIBG) performed at 6 weeks after the operation showed moderate MIBG uptake in a new 1 cm node medial to the IVC, three discrete MIBG-avid metastases identified in the liver and marked MIBG uptake in lytic lesion within C6 vertebral body, which was FDG negative. He received two doses of therapeutic I131 MIBG to control bone and lung metastases. The patient is alive with controlled metastatic disease over 4 years after his initial presentation.
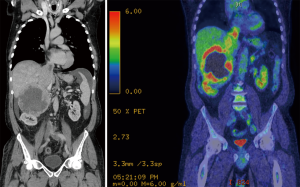
These figures demonstrate encouraging outcomes in patients otherwise deemed inoperable and highlight the importance of centralising adrenal surgery in a small number of units with adequate multidisciplinary support.
Conclusions
We described the surgical technique of using CPB associated with DHCA for the treatment of adrenal tumours extending into the RA. Establishing a dedicated multidisciplinary team with experience in managing these challenging cases is fundamental to offer treatment to patients with advanced disease, who would otherwise risk being turned down for surgery. A close collaboration between general and cardiac surgeons and a deep understanding of the surgical procedure steps are fundamental to safely performing these procedures.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mansmann G, Lau J, Balk E, et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev 2004;25:309-40. [Crossref] [PubMed]
- Ekici S, Ciancio G. Surgical management of large adrenal masses with or without thrombus extending into the inferior vena cava. J Urol 2004;172:2340-3. [Crossref] [PubMed]
- Osman Y, Haraz A, El-Mekresh M, et al. Adrenal tumors with venous thrombosis: a single-institution experience. Urol Int 2011;87:182-5. [Crossref] [PubMed]
- Chiche L, Dousset B, Kieffer E, et al. Adrenocortical carcinoma extending into the inferior vena cava: presentation of a 15-patient series and review of the literature. Surgery 2006;139:15-27. [Crossref] [PubMed]
- Nesbitt JC, Soltero ER, Dinney CP, et al. Surgical management of renal cell carcinoma with inferior vena cava tumor thrombus. Ann Thorac Surg 1997;63:1592-600. [Crossref] [PubMed]
- Delis SG, Bakogiannis A, Ciancio G, et al. Surgical management of large adrenal tumours: the University of Miami experience using liver transplantation techniques. BJU Int 2008;102:1394-9. [PubMed]
- Yan TD, Bannon PG, Bavaria J, et al. Consensus on hypothermia in aortic arch surgery. Ann Cardiothorac Surg 2013;2:163-8. [PubMed]
- McCullough JN, Zhang N, Reich DL, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg 1999;67:1895-9; discussion 1919-21.
- Mihai R, Iacobone M, Makay O, et al. Outcome of operation in patients with adrenocortical cancer invading the inferior vena cava--a European Society of Endocrine Surgeons (ESES) survey. Langenbecks Arch Surg 2012;397:225-31. [Crossref] [PubMed]


