
How to assess a CTA of the abdomen to plan an autologous breast reconstruction
Introduction
Autologous breast reconstruction using abdominal based flaps represent an integral component of recovery to breast cancer patients. The most commonly utilised abdominal flaps include the transverse rectus abdominis musculocutaneous (TRAM) flap, the deep inferior epigastric perforator (DIEP) flap and the superficial inferior epigastric artery (SIEA) flap. Currently, the DIEP flap is the preferred option among both surgeon and patient alike due to its aesthetically similar appearance, contour and texture to breast tissue and low donor site morbidity (1).
Anatomy
An understanding of the anatomy of the deep inferior epigastric artery (DIEA) is clearly essential when performing a DIEP flap. The DIEA has classically been described with three branching patterns: type I displaying a single trunk, type II with a bifurcating DIEA and type III with a trifurcating DIEA (2,3) (Figure 1). These branches distribute 5–6 major perforators to the muscle and overlying subcutaneous tissues (3). The perforators have a tortuous intramuscular course ranging from short to long through the rectus muscle (4) (Figure 2). These perforators can be further categorised into a medial and lateral row. Medial row perforators have a larger internal diameter, a direct course to Scarpa’s fascia and a greater branching pattern crossing the midline of the abdomen (5). Contrastingly, lateral row perforators have a smaller internal diameter, a transverse course to Scarpa’s fascia, less branching and doesn’t tend to cross the midline (5). This has led to separate categorisations of ‘perforator angiosomes’, for each of medial and lateral row perforators (Figure 3).

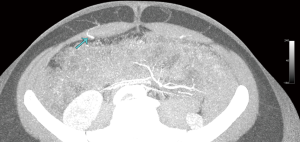
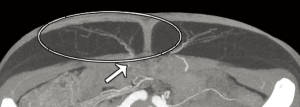
Computed tomographic angiography (CTA)
The vascular anatomy of the abdomen displays significant inter-individual variability and therefore it is common practice to utilise imaging to aid in operative planning. Modern imaging technologies such as CTA has become the mainstay of pre-operative planning due to its ability to map out the vascular anatomy (6,7). CTA has introduced an era of “personalised reconstructive surgery” with the added benefit of reducing post-operative complications such as fat necrosis and donor site morbidity in DIEP flaps (8).
‘Our’ technique for utilizing CTA is described in the following manuscript and digital video, demonstrating the anatomy, methodology and approach to interpretation of CTA for DIEP flaps (see Figure 4).

Methods
Equipment
CT hardware
- Siemens SOMATOM Sensation 64 multi-detector row CT scanner (Siemens Medical Solutions, Erlangen, Germany);
- One hundred mL of intravenous contrast (Omnipaque 350; Amersham Health, Princeton, USA).
CT software
- Free software: Horos™ (The Horos Project, Nimble Co LLC Purview, Annapolis, MD, USA);
- Commercial software: Osirix™ (Pixmeo, Geneva, Switzerland), Siemens™ Syngo Inspace 4D (Version 2006A; Siemens, Berlin, Germany).
3D reconstruction
3D reconstructions are configured with volume-rendering technique (VRT) and maximum intensity projections (MIPs). The colour look-up table (CLUT) function is used to perform VRT reconstruction. A range of CLUT variations are suitable, and have been published to date. VRT imaging demonstrates perforator size and location by highlighting their subfascial and subcutaneous course as they emerge from the rectus sheath, while MIP demonstrates the intramuscular course of the perforators (10).
Reporting
CTA reporting for the DIEP flap requires consideration of seven main areas:
- Perforator size and location;
- Perforator angiosome;
- Intramuscular course;
- DIEA pedicle;
- Venous anatomy;
- SIEA and superficial inferior epigastric vein (SIEV);
- Abdominal wall structure.
(I) Perforator size and location
Perforators with a diameter greater than 0.5 mm are noted at their point of emergence from the anterior rectus sheath and plotted on VRT reconstructions. The largest perforator is then localised in relation to the umbilicus and the transverse and caudo-cranial distances are recorded. Ideally perforators with a diameter greater than 1 mm is preferred as they are easier to dissect and more resilient (11).
(II) Perforator angiosome
Once identifying perforators of an adequate size, review axial slices of the CTA to demonstrate its branching pattern or “perforator angiosome”. This is a crucial step in designing the DIEP flap as tissue outside of the perforator angiosome should be discarded. To enhance the size of the harvested flap, a combination of two or more perforator angiosomes can be utilised.
(III) Intramuscular course
The next step is to review the intramuscular course of the larger perforators. Ideally to reduce abdominal site complications it is preferable to choose a perforator with a short course through the rectus muscle as this is associated with less dissection and therefore reduced donor site complications.
(IV) DIEA pedicle
The DIEA pedicle is identified as it originates from the external iliac artery and tracked to determine its branching pattern of either type I, type II or type III. A type I or type II DIEA branching pattern is associated with a shorter intramuscular course (3). A type III branching pattern tends to traverse a longer intra-muscular distance and should be avoided. When a type III branching pattern is encountered it is preferable to utilise perforators from the contralateral DIEA or even to consider a TRAM flap if suitable (3).
(V) Venous anatomy
Once the arterial vascular anatomy has been established the surgeon should then consider the venous anatomy. Venous drainage represents a significant portion of vascular complications encountered by the reconstructive surgeon of the breast (12,13). The abdominal venous anatomy contrasts to its arterial counterpart by having a dominant superficial drainage system compared to a dominant deep arterial system (Figure 5). The method used for mapping the arterial perforators is duplicated to identify the size and location of the superficial and deep venous systems. The venae comitantes of the DIEA is reliably identified running alongside the artery within or deep to the rectus muscle and then turns laterally with the DIEA to reach the femoral vein (14).
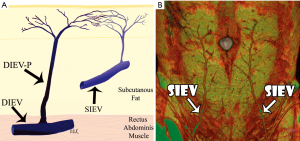
(VI) SIEA and SIEV
A holistic approach to the DIEP flap includes reviewing the SIEA and SIEV. The same technique is implemented to identify and assess the diameter and location of the SIEA. This is especially beneficial when the DIEA perforators have a long intramuscular course and smaller diameter indicating a technically difficult and risky flap elevation. Consideration of the SIEA enables the surgeon to plan prospectively if an SIEA flap would be better suited based on the patient’s vascular anatomy. The SIEV is assessed for its location, size and length for a SIEA flap and in cases where a secondary venous outlet is required for the DIEP flap. Reconstructive breast surgeons consider the SIEV as the donor vein of choice because it provides drainage through deep and superficial venous territories (14).
(VII) Abdominal wall structure
Finally, the abdominal wall structure should be assessed specifically for abdominal wall herniation and rectus divarication. These weak points in the abdominal wall can be addressed intra-operatively during sheath closure. Review of the rectus sheath also enables planning of sheath incisions in relation to the perforator course to minimise the risk of donor site complications.
Clinical application
The pre-operative plan formulated from CTA review can be implemented in the operating theatre. The first step is to mark out the chosen perforator(s) on the patient’s abdomen with a surgical marker. A Doppler ultrasound can supplement localisation of perforator(s). More recently, a “perforasome template” of the DIEA can be 3D printed utilising images from the patient’s CTA to further enhance flap design preoperatively (15).
Intra-operatively, knowledge of the intramuscular course of the perforator will aid dissection and reduce error and operative time (8). Once the chosen perforators are located and dissected down to the DIEA pedicle, the perforator angiosome is estimated based on the CTA and areas with poor perfusion are discarded. At this point, the SIEV if subjectively engorged or there are signs of venous congestion in the flap should be utilised as secondary venous drainage. In most cases, a contralateral SIEV is preferred (14). Finally, weak points in the abdominal wall identified on the CTA can be reinforced prior to donor site closure.
Conclusions
CTA has become a mainstay of pre-operative planning in the DIEP flap due to ease of access, affordability, reproducibility and operator independence (16). Extensive research has demonstrated that CTA has close to 100% sensitivity and specificity in detecting perforators (16). However, this must be balanced with the higher exposure to ionising radiation, sensitivity to contrast media and contrast induced-nephrotoxicity when compared to MRA or ultrasound. The ionising radiation dose can now be reduced to 5 mSv, equivalent to two abdominal X-rays, with appropriate software and hardware modifications (6,17). The optimal CTA parameters include a supine position, assessment of flap area only, instilling a bolus of contrast at the common femoral artery and caudo-cranial scanning in the direction of DIEA flow with acquisition time set to 4 seconds (18).
MRA is a strong contender against CTA as it has no radiation exposure and a safer contrast allergy profile. MRA provides clearer definition of the intra-muscular course of the perforators while CTA is superior at demonstrating the subcutaneous course (16). MRA has had limited uptake in the clinical setting due to high average costs, susceptibility to motion artefact, contraindication with MRI incompatible devices and prolonged examination window, however certainly has an evolving role.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent for all clinical or imaging reproductions was obtained.
References
- Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32:32-8. [Crossref] [PubMed]
- Moon HK, Taylor GI. The vascular anatomy of rectus abdominis musculocutaneous flaps based on the deep superior epigastric system. Plast Reconstr Surg 1988;82:815-32. [Crossref] [PubMed]
- Rozen WM, Palmer KP, Suami H, et al. The DIEA branching pattern and its relationship to perforators: the importance of preoperative computed tomographic angiography for DIEA perforator flaps. Plast Reconstr Surg 2008;121:367-73. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Pan WR, et al. Raising perforator flaps for breast reconstruction: the intramuscular anatomy of the deep inferior epigastric artery. Plast Reconstr Surg 2007;120:1443-9. [Crossref] [PubMed]
- Rozen WM, Ashton MW, Le Roux CM, et al. The perforator angiosome: a new concept in the design of deep inferior epigastric artery perforator flaps for breast reconstruction. Microsurgery 2010;30:1-7. [PubMed]
- Clavero JA, Masia J, Larranaga J, et al. MDCT in the preoperative planning of abdominal perforator surgery for postmastectomy breast reconstruction. AJR Am J Roentgenol 2008;191:670-6. [Crossref] [PubMed]
- Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7. [Crossref] [PubMed]
- Fitzgerald O'Connor E, Rozen WM, Chowdhry M, et al. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland surgery 2016;5:93-8. [PubMed]
- Rozen WM, Bhullar HK, Hunter-Smith D. Video demonstrating ‘Our’ technique for utilizing computed tomographic angiography (CTA), highlighting the anatomy, methodology and approach to interpretation of CTA for deep inferior epigastric artery perforator (DIEP) flaps. Asvide 2019;6:300. Available online: http://www.asvide.com/watch/32985
- Rozen WM, Phillips TJ, Stella DL, et al. Preoperative CT angiography for DIEP flaps: 'must-have' lessons for the radiologist. J Plast Reconstr Aesthet Surg 2009;62:e650-1. [Crossref] [PubMed]
- Rozen WM, Ashton MW. Modifying techniques in deep inferior epigastric artery perforator flap harvest with the use of preoperative imaging. ANZ J Surg 2009;79:598-603. [Crossref] [PubMed]
- Blondeel PN. One hundred free DIEP flap breast reconstructions: a personal experience. Br J Plast Surg 1999;52:104-11. [Crossref] [PubMed]
- Blondeel PN, Arnstein M, Verstraete K, et al. Venous congestion and blood flow in free transverse rectus abdominis myocutaneous and deep inferior epigastric perforator flaps. Plast Reconstr Surg 2000;106:1295-9. [Crossref] [PubMed]
- Rozen WM, Ashton MW. The venous anatomy of the abdominal wall for Deep Inferior Epigastric Artery (DIEP) flaps in breast reconstruction. Gland Surg 2012;1:92-110. [PubMed]
- Chae MP, Hunter-Smith DJ, Rostek M, et al. Enhanced Preoperative Deep Inferior Epigastric Artery Perforator Flap Planning with a 3D-Printed Perforasome Template: Technique and Case Report. Plast Reconstr Surg Glob Open 2018;6:e1644. [Crossref] [PubMed]
- Chae MP, Hunter-Smith DJ, Rozen WM. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland surgery 2015;4:164-78. [PubMed]
- Masia J, Clavero JA, Larranaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg 2006;59:594-9. [Crossref] [PubMed]
- Phillips TJ, Stella DL, Rozen WM, et al. Abdominal wall CT angiography: a detailed account of a newly established preoperative imaging technique. Radiology 2008;249:32-44. [Crossref] [PubMed]


