Update on the surgical management of Paget’s disease
What is Paget’s disease?
Mammary Paget’s disease is a rare form of breast neoplasm that is associated with approximately 3% of all breast cancers (1). It is much more frequently found in females than males, though it can be found in men, with some series suggesting worse prognosis in men (2). Velpeau described the eczematous lesions of Paget’s disease in 1856 (3), but it was Sir James Paget who first described the association with underlying breast cancer in 1874 (4). In his paper, Paget described 15 women between 40 and 60 years old, who first presented with the skin changes involving nipple-areolar complex, and subsequently progressed to development of breast cancer. He noted that all fifteen patients initially presented with an itching eczema-like rash and discharge from the nipple, which were refractory to common remedies, and within the following year progressed to cancer.
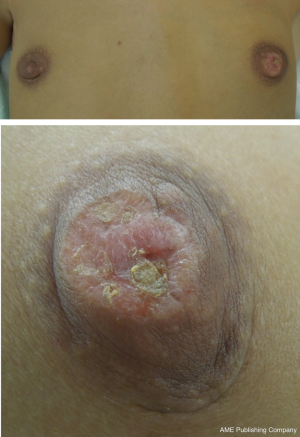
The vast majority of patients diagnosed with Paget’s disease have an associated underlying neoplasm in the breast. In several series, the percentage of patients diagnosed with Paget’s disease of the nipple found to have invasive or non-invasive carcinoma is upwards of 90% (1,5-8). Up to 50% of the patients with Paget’s disease present with a palpable mass in their breast (1,6). Most often Paget’s disease is diagnosed in women in the sixth and seventh decade with a mean age of diagnosis reported at 62.6 years (9), but the age distribution is equivalent to that of breast cancer in general, and it may affect younger patients or octogenarians (10). The early presentation is as Paget described-an itchy, eczema-like rash involving the nipple-areolar complex (Figure 1). It may be associated with a dry exudate and sometimes spreads to other parts of the breast. In more advanced stages of the disease the lesions may progress to ulceration and cause nipple retraction or bloody discharge from the nipple (11-13).
On the microscopic level, Paget’s disease is characterized by epidermal invasion by malignant glandular cells, which are large, foamy cells that may contain mucin (13,14). These cells when stained with hematoxylin and eosin have pale cytoplasm and hyperchromatic nuclei (Figure 2) (15). In more advanced disease keratinocytes with atypia and epidermal hyperplasia are not uncommon. Some cells might also contain melanin in their cytoplasm (11).

Two theories have been proposed in regards to pathogenesis of Paget’s disease: (I) epidermotropic theory and (II) in situ malignant transformation theory. The first theory claims that changes typical for Paget’s disease arise in the ductal cells, and spread along the basement membrane to the nipple (16). This theory is supported by the fact that most patients with Paget’s have underlying breast cancer, and the cells from the nipple are histologically similar to the associated invasive carcinoma (17). The in situ malignant transformation theory claim holds that Paget’s disease originates in the epidermal cells of the nipple by malignant transformation of keratinocytes and is not associated with any coexisting neoplastic process in the affected breast (18,19).
The diagnostic challenge of Paget’s disease
Sometimes the diagnosis of Paget’s disease is delayed because the lesions are mistaken for benign skin conditions such as contact dermatitis, duct ectasia, or nipple discharge resulting from ductal papilloma. Ulcerated Paget’s may resemble basal cell carcinoma and in the case of pigmented lesions, melanoma should be included in the differential diagnosis (13,14).
In any patient presenting with an itching or ulcerated lesion of the nipple, a tissue biopsy should be obtained to exclude the diagnosis of Paget’s disease. This can be successfully accomplished with a skin punch biopsy. Once a patient has a confirmed diagnosis of Paget’s disease, further imaging should be undertaken to evaluate the full extent of the disease, since Paget’s disease is often associated with malignancy that could be multicentric (defined as two or more foci in separate anatomical quadrants of the breast) or multifocal (multiple foci of carcinoma found in the same quadrant) (20).
Since ultrasound and mammography are not specific for detection of multifocal and/or multicentric disease, Siponem et al. have investigated the role of MRI in evaluating patients with the diagnosis of Paget’s disease. In their retrospective review of 58 patients, the authors found mammogram and ultrasound to be 79% and 74% sensitive respectively in detecting invasive cancer. These numbers were lower for underlying ductal carcinoma in situ (DCIS) (39% and 19% respectively). MRI, on the other hand, was 100% sensitive for infiltrating carcinoma and 44% for DCIS (21). Dixon et al. in their series of 48 women advised against use of mammography to evaluate patients with Paget’s disease. In their experience, mammography failed to reveal radiological evidence of underlying disease in 43% of patients with carcinoma confirmed by histology (22).
Morrogh with her colleagues looked at 34 women who presented with nipple complaints and were subsequently found to have Paget’s disease on biopsy. In this study, 94% of patients were found to have underlying breast malignancy. All patients were evaluated with diagnostic mammograms as first-line imaging, with 32% of the films read as suspicious for malignancy, while the remaining films were read as negative. Half of the patients with BIRADS ≥4 were further evaluated with MRI, but their subsequent management was not influenced by MRI results. Out of 23 patients with negative mammograms, 15 proceeded straight to surgery and only one of them was found not to have an associated breast malignancy. The remaining 8 patients were evaluated with MRI, half of which were BIRADS ≥4, and the other half was successfully treated with breast conserving therapy based on MRI findings of unifocal disease. Overall 57% of cancers diagnosed with MRI were missed on mammography. The authors concluded that MRI is highly sensitive for detection of malignancies occult on mammography and greatly aids pre-operative planning (23).
What is the appropriate surgical approach to Paget’s disease?
Because of the rare incidence of Paget’s disease, there are no randomized studies evaluating the optimal treatment strategy of patients affected by it, but several retrospective reviews are available. Historically, patients with diagnosis of Paget’s disease have been treated with mastectomy, because of a high likelihood of association with multicentric and/or multifocal breast cancer.
Kothari and colleagues in their retrospective review of a series of 70 women with a diagnosis of Paget’s disease found that almost 60% of the patients had underlying invasive carcinoma. All patients in this study underwent total mastectomy. The results of pathological analysis were then correlated with patients’ original presentation and diagnostic imaging in order to determine whether a cure could be attained with a less invasive operation. They reported that more than a third of the patients in their study group had a palpable mass on presentation; Of those 41% had multifocal disease and 30% had multicentric disease. Among the remaining patients without a palpable mass, more than 60% had multifocal/multicentric disease. Overall, more than 60% of the patients had axillary nodal involvement. The authors concluded that 75% of the patients under investigation would be treated inadequately had they undergone breast preserving surgery consisting of wide local excision of the nipple-areolar complex and excision of the underlying tissue only (24).
As a breast conserving therapy has emerged to become the most common treatment of invasive breast cancer, it has become more common in the management of mammary Paget’s. Dominici et al. retrospectively reviewed 51 patients with Paget’s disease over a period of 12 years. All of the patients were evaluated with imaging. MRI found radiographic abnormalities in 78% of the patients, while only 47% of the patients had positive findings on a mammogram. 23% of patients had a palpable mass with further workup positive for invasive cancer. All of these patients underwent total mastectomy. Altogether 37% of patients had a breast-conserving surgery with central lumpectomy, with the majority receiving adjuvant radiation afterwards. During a 29-month period of follow-up, only one patient in the breast conservation group and one patient after mastectomy had distant metastases and none had local recurrence. The group concluded that breast conserving therapy is effective in patients with limited disease proved by imaging (5).
Dalberg and her colleagues looked retrospectively at a population cohort of more than 200 women with mammary Paget’s disease over a period of 25 years. 20% of the patients were treated with local surgery, while the rest underwent total mastectomy. On average the subjects were followed for 10 years. Univariate analysis showed that the type of surgery performed had no influence on breast cancer or Paget’s disease free survival. The only two significant risk factors for recurrence or death were underlying invasive cancer and the presence of a palpable mass on presentation. The authors concluded that in carefully selected patients with Paget’s disease, breast conserving surgery may have similar results to total mastectomy (15).
One prospective study of 61 patients with Paget’s disease without underlying invasive carcinoma looked at local recurrence following breast conserving therapy and radiotherapy. DCIS was the histological diagnosis in 93%, while the remaining patients had Paget’s of the nipple only. All were treated with cone excision of the nipple-areolar complex and underlying breast tissue followed by whole-breast irradiation. At the conclusion of the study, with median follow-up of 75 months, only 7% of patients had a local recurrence, 75% of which were invasive cancers. This study further underscores the feasibility of breast conserving surgery for Paget’s (25).
Though nodal evaluation is necessary in patients with invasive breast cancer, the role for sentinel lymph node biopsy is not well established in management of Paget’s disease. Several studies have retrospectively attempted to characterize the utility of SLN biopsy in DCIS or invasive cancer in the setting of Paget’s disease as well as in Paget’s disease alone. Laronga et al. in 2006 published a review of their database of 54 patients with Paget’s disease, 18 of whom had received no axillary staging or a complete axillary dissection, and 36 who were evaluated with sentinel lymph node biopsy (26). Invasive cancer was present in 26% of their patients, with isolated Paget’s disease present in 33%, a higher percentage than most series, and 41% of patients had histological evidence of DCIS. There was no significant difference in outcomes between groups treated with SLN biopsy or the “no SLN biopsy group” that included both ALND and patients without any evaluation of the axilla (9 patients). Overall 11% of their patients had sentinel lymph node involvement, all of which were in the subset with invasive cancer. Sukumvanish et al., in a 2007 retrospective review of 39 cases of Paget’s disease treated with sentinel lymph node biopsy, demonstrated a 27% rate of underlying invasive carcinoma in patients (a subset of 19 patients) without radiographic or clinical findings suggestive of invasive disease, and 11% of those patients went on to have positive sentinel nodes. Consistent with many prior series, 90% of their patients had an underlying malignancy. Overall, 28% of Paget’s patients had positive sentinel nodes (27).
The applicability of their findings in the subset of patients without radiological evidence for further disease is uncertain today as MRI is becoming the diagnostic standard in Paget’s. Given the demonstrated sensitivity of MRI in the diagnosis of invasive breast cancer, consideration for sentinel lymph node biopsy should be given to any patient without clinical or radiological evidence of nodal involvement undergoing mastectomy and to any patient with MRI evidence for invasive disease or biopsy proven invasive disease that is being planned for breast conserving therapy. The utility of sentinel node biopsy in patients without invasive disease—in suspected isolated Paget’s or DCIS—is not presently clear.
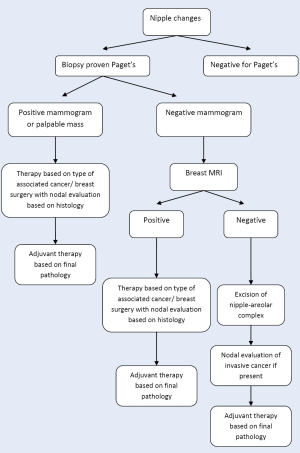
Based upon the available data, we propose the following algorithm in evaluation and treatment of patients with Paget’s disease (Figure 3). Any patient presenting with nipple changes suspicious for Paget’s disease, should undergo biopsy of the lesion. If the results are not consistent with Paget’s, the appropriate treatment should be instituted based on pathological diagnosis. If the biopsy is positive, mammography should be performed. In the case of negative or inconclusive mammogram, the patient should be further evaluated with breast MRI. The patients with negative MRI but with Paget’s in the nipple should undergo excision of the nipple-areolar complex followed by adjuvant radiation. Those with palpable mass, positive mammogram or MRI with evidence of malignancy should undergo therapy based on the type and extent of associated cancer, including further biopsies, partial or total mastectomy and nodal evaluation if indicated.
Summary
Paget’s disease of the breast is a relatively uncommon condition affecting the nipple and the surrounding tissue. Almost universally, it is associated with DCIS or invasive cancer in the ipsilateral breast. Because these underlying malignancies tend to be multifocal and multicentric, mammography often is not sufficient and MRI may be necessary to evaluate the true extent of the disease. Both total mastectomy and breast preserving surgery followed by appropriate adjuvant therapy are acceptable treatment options for carefully selected patients with Paget’s disease.
Acknowledgements
The authors thank Yanping Li MD, PhD, Department of Pathology VCUHS, for providing the representative pathological pictures. Kazuaki Takabe is funded by United States National Institute of Health (R01CA160688) and Susan G. Komen for the Cure [Investigator Initiated Research Grant (IIR12222224)].
Disclosure: The authors declare no conflict of interest.
References
- Ashikari R, Park K, Huvos AG, et al. Paget’s disease of the breast. Cancer 1970;26:680-5. [PubMed]
- Desai DC, Brennan EJ Jr, Carp NZ. Paget’s disease of the male breast. Am Surg 1996;62:1068-72. [PubMed]
- Velpeau A. eds. On disease of the mammary areola preceding cancer of the mammary region. London: Sydenham Society, 1856.
- Paget J. On disease of the mammary areola preceding cancer of the mammary gland. St Barts Hospital Rep 1874;10:87-9.
- Dominici LS, Lester S, Liao GS, et al. Current surgical approach to Paget’s disease. Am J Surg 2012;204:18-22. [PubMed]
- Chaudary MA, Millis RR, Lane EB, et al. Paget’s disease of the nipple: a ten year review including clinical, pathological, and immunohistochemical findings. Breast Cancer Res Treat 1986;8:139-46. [PubMed]
- Caliskan M, Gatti G, Sosnovskikh I, et al. Paget’s disease of the breast: the experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat 2008;112:513-21. [PubMed]
- Yim JH, Wick MR, Philpott GW, et al. Underlying pathology in mammary Paget’s disease. Ann Surg Oncol 1997;4:287-92. [PubMed]
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the U.S. Cancer 2006;107:1448-58.
- Nance FC, DeLoach DH, Welsh RA, et al. Paget’s disease of the breast. Ann Surg 1970;171:864-74. [PubMed]
- Karakas C. Paget’s disease of the breast. J Carcinog 2011;10:31. [PubMed]
- Kollmorgen DR, Varanasi JS, Edge SB, et al. Paget’s disease of the breast: a 33-year experience. J Am Coll Surg 1998;187:171-7. [PubMed]
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol 2007;21:581-90. [PubMed]
- Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol 2000;53:742-9. [PubMed]
- Dalberg K, Hellborg H, Wärnberg F. Paget’s disease of the nipple in a population based cohort. Breast Cancer Res Treat 2008;111:313-9. [PubMed]
- Muir R. The pathogenesis of Paget’s disease of the nipple and associated lesions. Br J Surg 1935;22:728-37.
- Inglis K. Paget’s disease of the nipple; with special reference to the changes in the ducts. Am J Pathol 1946;22:1-33.
- Yim JH, Wick MR, Philpott GW, et al. Underlying pathology in mammary Paget’s disease. Ann Surg Oncol 1997;4:287-92. [PubMed]
- Paone JF, Baker RR. Pathogenesis and treatment of Paget’s disease of the breast. Cancer 1981;48:825-9. [PubMed]
- Fu W, Mittel VK, Young SC. Paget disease of the breast: analysis of 41 patients. Am J Clin Oncol 2001;24:397-400. [PubMed]
- Siponen E, Hukkinen K, Heikkilä P, et al. Surgical treatment in Paget’s disease of the breast. Am J Surg 2010;200:241-6. [PubMed]
- Dixon AR, Galea MH, Ellis IO, et al. Paget’s disease of the nipple. Br J Surg 1991;78:722-3. [PubMed]
- Morrogh M, Morris EA, Liberman L, et al. MRI identifies otherwise occult disease in select patients with Paget disease of the nipple. J Am Coll Surg 2008;206:316-21. [PubMed]
- Kothari AS, Beechey-Newman N, Hamed H, et al. Paget disease of the nipple: a multifocal manifestation of higher-risk disease. Cancer 2002;95:1-7. [PubMed]
- Bijker N, Rutgers EJ, Duchateau L, et al. Breast-conserving therapy for Paget disease of the nipple: a prospective European Organization for Research and Treatment of Cancer study of 61 patients. Cancer 2001;91:472-7. [PubMed]
- Laronga C, Hasson D, Hoover S, et al. Paget’s disease in the era of sentinel lymph node biopsy. Am J Surg 2006;192:481-3. [PubMed]
- Sukumvanich P, Bentrem DJ, Cody HS 3rd, et al. The role of sentinel lymph node biopsy in Paget’s disease of the breast. Ann Surg Oncol 2007;14:1020-3. [PubMed]