
Shear wave elasticity of breast lesions: would it be correlated with the extracellular matrix components?
Introduction
Elastography is a new imaging technology which quantifies the “stiffness” of breast lesions (1,2), and it can be performed during routine breast ultrasonographic (US) examinations. Studies had shown that one of the characteristics of cancer was the increase in matrix stiffness, and stiffness had been applied in the cancer detection (3,4).
Previous studies had demonstrated that shear wave elastography (SWE) could give reproducible and quantitative information about solid breast lesions, and the diagnostic accuracy of which was at lowest as good as traditional ultrasound with BI-RADS classification (5,6). Evans A demonstrated that the prognosis of breast lesion with a higher mean rigidity values at SWE was poorer (7). Compared with benign breast lesions, although malignant breast lesions were stiffer, the reasons for this discrepancy still remained to be unraveled.
The extracellular matrix (ECM) is a complex network composed of glycoproteins and proteoglycans which determines the structure of tissues and is a condition for many biological activities. Studies suggested that ECM stiffening was correlated with tumor invasions and metastasis (8). Levental et al. (9) reported that the occurrence of breast tumorigenesis was associated with collagen crosslinking, ECM sclerosis, and increased focal adhesions. The ECM is mainly comprised of interstitial collagens (mainly type I and type III collagen), elastic fiber, fibronectin (FN) and multifarious proteoglycans. An excessive accumulation of ECM components including different types of collagen, FN, elastic fiber, laminin (LN) and proteoglycans is observed in breast carcinoma (10,11). In our previous study, we demonstrated that the content of collagen fiber was closely correlated with the elasticity of breast lesions, which might play an important role in stiffness of breast lesions (12). As far as we know, the correlation between the elasticity and ECM components in breast lesions have not been systematically reported.
Thus, this study aims to study the correlation between shear wave elasticity and ECM components in breast lesions.
Methods
Patients
Between March 2012 and March 2013, a prospective study was performed at our department. The study population included 65 patients referred to our department for ultrasound-guided vacuum-assisted biopsy (VAB) of a breast lesion that was sonographically apparent. Subjects were included according to their consent for the following testing. Patients would be excluded if they (I) were lactating or pregnant, (II) with breast implants, (III) were receiving radiotherapy or chemotherapy for skin masses, any cancer, or any lesions which had been biopsied, (IV) had a surgery on the ipsilateral breast. Pathologic diagnosis was taken as the golden criterion.
In the 65 female patients (age, 38–71 years; mean age ± standard deviation, 48.8±21.3 years), 2 women presented with nipple discharge, and 45 exhibited with a palpable mass. The maximum diameter of the 69 breast nodules was 0.7–4.6 cm (mean diameter ± standard deviation: 2.1±2.0 cm).
Informed consent for subsequent examinations was obtained from all 65 patients, and our local Ethics Committee approved this study.
US examination and SWE examination
Aixplorer® ultrasound system (SuperSonic Imagine, Aix en Provence, France) was used for SWE examination and US examination with a probe of which the center frequency was 12 MHz.
The ultrasound images were classified by two breast radiologists with 10 and 15 years’ experience of breast ultrasound, respectively, according to American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS).
Two independent experts performed the SWE examination. The radiologists convert to the elastography modality after the ultrasound examination. The probe should be applied as lightly as possible to keep the lesion free from pressure. During the obtainment of the elastography image, the probe needs to remain stationary for 10–20 seconds (due to the slow frame rate), which was usually best done while holding the breath. The selected elastography views were those which showed the clearest abnormal stiffness in the plane, but without pressure or movement artifact, for example the red area below the probe. After obtaining the stable image, recording the image and choosing the region of interest (ROI) to calculate the value of elasticity. The ROI was chosen to cover the entire lesion as large as possible including possibly presented calcification and the margin of the lesion. Since the ROI box was circular, it was difficult to cover the entire lesion, especially the ones with irregular edge. But we did our best to include the lesion as much part as possible, especially the hardest part. Then another ROI was selected in peripheral parenchyma at a depth as similar as possible with the depth of the breast lesion. For every patient, three ROIs were selected from the lesion and the peripheral parenchyma, respectively, and the final value was expressed by the mean value. ROI in the peripheral parenchyma should be as consistent as possible with the corresponding breast lesion in depth and size. The maximum value, mean value, minimum value within the ROI, called max elasticity, mean elasticity and min elasticity, and elasticity ratio of lesions to peripheral parenchyma were recorded.
A blinded review was performed by two independent expert observers to the static images of every lesion.
Biopsy procedure
Vacuum-assisted biopsy (EnCor® MR, SenoRx, Allso Viejo, CA, USA) was routinely used. All biopsies were performed by two radiologists with 13 and 9 years of interventional experience, respectively. Obtaining two or three samples in the area of largest elasticity.
Van Gieson dye
Van Gieson dye was for the staining of collagen fiber. Deparaffinizing the slides and hydrated with distilled water, Weigert iron hematoxylin was used for stain for 10 minutes, and tap water was used for rinsing. Then 1% hydrochloric acid alcohol was used for a rapid differentiation, and after that, the slides were rinse for 2 minutes with running water. After using Van Gieson’s solution for Counterstain for 2 minutes, 95% alcohol was used for a quick differentiation of slides for a few seconds, and then 100% alcohol was used for dehydrating. After that, xylene was used for clearing for 3 minutes, resinous mounting medium was used for the coverslip of slides and then microscope was used for the observation of slides.
Aldehyde fuchsin staining
Aldehyde Fuchsin dye was for the staining of elastic fiber. Before and after the slides were bleached with acid bleach, using running water to wash the slides for 2 minutes. After 70% ethanol cleaning, the slides were dyed by aldehyde fuchsin, and then washed by 70% ethanol until no purple liquid elute. After washed in running water, the slides were dyed with orange G, washed again and dehydrated with 95% alcohol and observed under the microscope.
Immunohistochemistry
Immunohistochemistry was for the staining of FN and LN. Prior to final dissection and block selection, a neutral buffered formalin was used overnight to fix slides. Immunohistochemistry was performed using standard methodologies (13). Negative controls (without primary antibody) were applied for all staining procedures.
Quantification of collagen fiber and elastic fiber
The collagen fiber area was quantitatively analyzed by Image-Pro Plus 5.1 Software. A board-certified pathologist performed computer graphic analyze under detailed measurement settings, and randomly selected 5 fields under high magnification (×400) each slide and then calculated the mean value.
Statistical analyses
SPSS16.0, standard version (SPSS Inc., Chicago, IL, USA) was used for all analyses. We used descriptive statistics to characterize patient features at study entry. The elastic differences in elastic fiber areas and collagen fiber between malignant and benign breast lesions were assessed using the Student’s t-test. Categorical and continuous variables such as FN and LN expression were summarized with the χ2 test or Fisher exact test. The correlation between collagen fiber area and elasticity was analyzed with Pearson correlation analysis. P<0.05 was considered to show a statistically significant. The relationship between max elasticity and variables was modeled with Multiple linear regression analysis.
Variabilities in the elasticity parameters between the two readers were calculated with κ statistics. κ ≤0.20 was considered slight; 0.21≤ κ ≤0.40 was considered fair; 0.41≤ κ ≤0.60 was considered moderate; 0.61≤ κ ≤0.80 was considered substantial; and 0.81≤ κ ≤1.00 was considered almost perfect. One-way analysis of variance (ANOVA) was used to calculated the Intraclass correlation coefficients (ICC) to measure the reliability.
Results
Pathology
Among the 69 masses, malignant lesions were 37 (53.6%) and benign lesions were 32 (46.4%). Malignant lesions consisted of 25 invasive ductal carcinoma, 11 ductal carcinoma in situ (DCIS) and 1 invasive lobular carcinoma (ILC). The remaining benign lesions included 7 fibroadenosis, 21 fibroadenomas, 1 inflammation, and 3 papillomas.
Comparison of elasticity between malignant and benign lesions
The maximum, mean, minimum elasticity, and elasticity ratio of lesions to peripheral parenchyma of lesions of malignant and benign breast lesions were showed in Table 1. Benign lesions exhibited significantly lower maximum, mean elasticity, and elasticity ratio of lesions to peripheral parenchyma than malignant lesions.
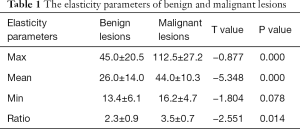
Full table
Comparison of ECM components between benign and malignant lesions
The mean areas of elastic fiber and collagen fiber of malignant and benign lesions could be seen in Table 2. Malignant lesions exhibited significantly larger mean collagen fiber area and elastic fiber area than benign lesions (Table 2, Figures 1,2).
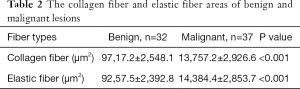
Full table
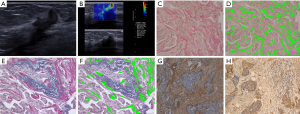
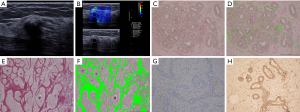
FN and LN can be expressed both in stromal cells and in tumor cells. The expressions of FN and LN in benign and malignant tumors could be seen in Table 3. All malignant tumors had FN expression in stromal cells, and the stromal cells of malignant lesions showed a significantly higher FN expression than those of benign lesions (P<0.05). FN expression in benign and malignant lesion cells was not significantly different.

Full table
On the contrary, the expression of LN in lesion cells and stromal cells between benign and malignant lesions was not significantly different.
Correlation between elasticity and ECM components
Simple linear regression analysis showed a significantly positive correlation between maximum elasticity and the contents of collagen fiber and elastic fiber, respectively (r=0.713, r=0.746, P<0.001) (Figure 3). There was no correlation between the expressions of FN and LN and elasticity.
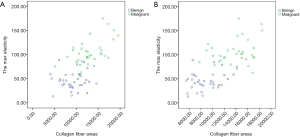
Multiple linear regression analysis showed that collagen fiber and elastic fiber were independent variables correlated with the maximum elasticity of breast lesions (r2=0.564, P=0.014).
Reproducibility
Reliability of the quantitative elasticity measurements on the SWE images was very favorable for the measurement of maximum and mean elasticity values. Maximum, mean elasticity and elasticity ratio of lesions to peripheral parenchyma of lesions were both reliable measured values, with ICC =0.82, 0.87, 0.79, respectively.
Interobserver agreements between the two radiologists for final assessment of elasticity parameters were similar, with κ =0.625±0.209.
Discussion
ECM is a complex network composed of proteoglycans and glycoproteins which determines the structure of tissues and is a condition for many biological activities. The components of ECM provide a great number of specific signals which directly affect cell growth, biosynthetic, morphology, differentiation, migration, and proliferation activities (14,15).
The most major structural protein in ECM is Collagen fiber (16), and it had been found that the increase of collagen fiber I could promote the formation, invasion, and metastasis of breast cancers (17,18). Mammography, which examined dense fibroglandular tissue of the breast, indicated a 4.64-fold increase in the risk of developing breast cancer in women who showed high breast density. Levental et al. reported that the occurrence of breast tumors was accompanied by ECM stiffening, collagen crosslinking, and increased focal adhesions. Induction of collagen crosslinking could promote focal adhesions, stiffened the ECM, induced oncogene-induced epithelial cell invasion and enhanced the activity of PI3 kinase (PI3K) (9). Studies had also shown that the strongest pathological factor determining the mean hardness of hepatocellular carcinomas was the proportion of areas of collagen fiber (19). This study exhibited that the mean area of collagen fiber in benign breast lesions was significantly smaller than that of malignant lesions, which indicated that the content of collagen fiber might participate in the progression of breast carcinoma. Also, this study demonstrated that the mean collagen fiber area was correlated with the maximum elasticity of breast lesions, which suggested that collagen fiber might participate in the formation of tumor stiffness.
Literatures demonstrate that the protein of ECM can interact with cancer cells specifically, such as elastic fiber. Recent studies have gradually revealed the mechanism of elastic fiber in tumor ECM. Certain cancer cells can express lysyl oxydase and synthesize elastic fiber, which provides an explanation for the frequent emergence of elastic tissue in breast cancers and other tumors (20). Study also demonstrated the function of elastic fiber in the elastosis of breast cancer (21). The results of this study are consistent with the former research. This study showed that benign breast lesions had a significantly smaller mean elastic fiber area than malignant lesion, which indicated that the content of elastic fiber might participate in the progression of breast carcinoma. Also, this study demonstrated that the mean elastic fiber area was correlated with the maximum elasticity of breast lesions, which suggested that collagen fiber might participate in the formation of tumor stiffness.
LN, a major basement membrane component, believed to play an important role in some ECM-regulated activities observed on epithelia while supporting and inducing early tumor development and tumor initiation (22). The role of LN in matrix elasticity and the role of LN are associated with the formation of tubular and acinar structures in breast (and other) epithelial cells (23) and are associated with the maintenance and/or acquisition of epithelial cell polarity (24). Actually, laminin 1 is thought to be the reason for the “softness” of the mammary stroma. FN is a fibril-forming glycoprotein, and could be upregulated during fibrotic response or desmoplasia. FN is expressed by both cancer cells and cancer-associated fibroblasts (CAFs). Cancer cells expressed more FN can lead to increased migratory behavior (25). In mouse models, it has been reported that FN expression is induced by cytokines secreted by the primary tumor in the secondary organs of the mouse models, this process can produce a stromal niche which promotes metastasis, that is, a pre-metastatic niche (26). In general, the expression of FN in breast cancer is linked to poor clinical outcome. Some studies demonstrate that FN expressed by cancer cell, in particular, is associated with poor overall survival and metastasis free rate in breast cancer patients (27,28). This study demonstrated that there was no correlation between the tumor elastic modulus and the expression of LN and FN, which suggested that LN and FN might not attribute significantly to the stiffness of breast lesions. According to this study, FN and LN expressed in basement membrane or stromal cells illustrated great differences between benign and malignant breast tumors, which indicated that ECM components might work in different pathway during tumor proliferation. These interesting differences need for further study.
Our study had several limitations. First, there are so many components in the ECM that could not be studied totally. We then chose to study some ECM components which were either important structural components of ECM, or correlated closely with tumor metastasis, such as collagen fiber, elastic fiber, FN and LN. These components are all very important components in ECM and have close correlation with ECM stiffness or tumorgenesis. Second, the content of collagen fiber of different types in the included breast lesions was not detected. It has been reported that the main collagen fiber which prop up the breast stroma in breast cancer was type I collagen fiber (3,5), and the relationship between collagen fiber of different types and the breast lesions stiffness would remain to be further studied.
Generally, our research indicated that the components of elastic fiber and collagen fiber was positively correlated with the elasticity of breast lesions, which suggested that further study of the mechanism of ECM might provide a new method for the study of the elasticity of breast carcinoma.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation (81771832); the Capital characteristics of the fund (Z161100000516190); and the Military health issues (17BJZ34).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Informed consent for subsequent examinations was obtained from all 65 patients, and our local Ethics Committee approved this study.
References
- Krouskop TA, Wheeler TM, Kallel F, et al. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging 1998;20:260-74. [Crossref] [PubMed]
- Lerner RM, Huang SR, Parker KJ. "Sonoelasticity" images derived from ultrasound signals in mechanically vibrated tissues. Ultrasound Med Biol 1990;16:231-9. [Crossref] [PubMed]
- Clavel C, Birembaut P, Adnet JJ, et al. Breast carcinomas and the extracellular matrix. Ann Pathol 1988;8:107-13. [PubMed]
- Modesti A, D'Orazi G, Scarpa S, et al. Ultrastructural and immunoelectron microscopic study of the desmoplastic stroma in carcinoma of the breast. G Chir 1989;10:245-9. [PubMed]
- Bai M, Du L, Gu J, et al. Virtual touch tissue quantification using acoustic radiation force impulse technology: initial clinical experience with solid breast masses. J Ultrasound Med 2012;31:289-94. [Crossref] [PubMed]
- Wang ZL, Li JL, Li M, et al. Study of quantitative elastography with supersonic shear imaging in the diagnosis of breast tumours. Radiol Med 2013;118:583-90. [Crossref] [PubMed]
- Evans A, Whelehan P, Thomson K, et al. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res 2010;12:R104. [Crossref] [PubMed]
- Acerbi I, Cassereau L, Dean I, et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 2015;7:1120-34. [Crossref] [PubMed]
- Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009;139:891-906. [Crossref] [PubMed]
- Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast 2013;22 Suppl 2:S66-72. [Crossref] [PubMed]
- Jodele S, Blavier L, Yoon JM, et al. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev 2006;25:35-43. [Crossref] [PubMed]
- Wang ZL, Sun L, Li Y, et al. Relationship between elasticity and collagen fiber content in breast disease: a preliminary report. Ultrasonics 2015;57:44-9. [Crossref] [PubMed]
- Purdie CA, Jordan LB, McCullough JB, et al. HER2 assessment on core biopsy specimens using monoclonal antibody CB11 accurately determines HER2 status in breast carcinoma. Histopathology 2010;56:702-7. [Crossref] [PubMed]
- LaFlamme SE, Auer KL. Integrin signaling. Semin Cancer Biol 1996;7:111-8. [Crossref] [PubMed]
- Weaver VM, Fischer AH, Peterson OW, et al. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol 1996;74:833-51. [Crossref] [PubMed]
- Kolácná L, Bakesova J, Varga F, et al. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol Res 2007;56 Suppl 1:S51-60. [PubMed]
- Alowami S, Troup S, Al-Haddad S, et al. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res 2003;5:R129-35. [Crossref] [PubMed]
- Li T, Sun L, Miller N, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev 2005;14:343-9. [Crossref] [PubMed]
- Honjo M, Moriyasu F, Sugimoto K, et al. Relationship between the liver tissue shear modulus and histopathologic findings analyzed by intraoperative shear wave elastography and digital microscopically assisted morphometry in patients with hepatocellular carcinoma. J Ultrasound Med 2014;33:61-71. [Crossref] [PubMed]
- Jalkanen S, Salmi M. Cell surface monoamine oxidases: enzymes in search of a function. Embo J 2001;20:3893-901. [Crossref] [PubMed]
- Mera SL, Davies JD. Elastosis in breast carcinoma: I. Immunohistochemical characterization of elastic fibres. J Pathol 1987;151:103-10. [Crossref] [PubMed]
- Alcaraz J, Xu R, Mori H, et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. Embo j 2008;27:2829-38. [Crossref] [PubMed]
- Xu R, Spencer VA, Bissell MJ. Extracellular matrix-regulated gene expression requires cooperation of SWI/SNF and transcription factors. J Biol Chem 2007;282:14992-9. [Crossref] [PubMed]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, et al. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 2002;115:39-50. [PubMed]
- Tse JM, Cheng G, Tyrrell JA, et al. Mechanical compression drives cancer cells toward invasive phenotype. Proc Natl Acad Sci U S A 2012;109:911-6. [Crossref] [PubMed]
- Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438:820-7. [Crossref] [PubMed]
- Bae YK, Kim A, Kim MK, et al. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum Pathol 2013;44:2028-37. [Crossref] [PubMed]
- Fernandez-Garcia B, Eiro N, Marin L, et al. Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology 2014;64:512-22. [Crossref] [PubMed]


