
BI-RADS 4 breast lesions: could multi-mode ultrasound be helpful for their diagnosis?
Introduction
Breast cancer is the most common malignant tumor in women worldwide, and ultrasound (US) has been an important tool for screening of breast lesions in China. In 2013, the American College of Radiology (ACR) issued the fifth edition of the Breast Imaging Report and Data System (BI-RADS) (1) to standardize the risk evaluation of breast lesions. According to the ACR BI-RADS US lexicon, breast lesions of category 4 have a 3–94% probability of malignancy and were subcategorized into 4a, 4b and 4c. Category 4a lesions have a 3–10% probability of malignancy, category 4b 11–50%, and category 4c 51–94%. This means, BI-RADS category 4 lesions are associated with a highly variable rate of breast cancer, and with a high rate of benign (61.2%) (2), which might result in a high rate of unnecessary biopsy.
US is an important screening and diagnostic method for breast lesions. However, the manifestations of conventional gray scale images of BI-RADS 4 breast lesions tend to have a certain degree of overlapping and are sometimes difficult to identify (3,4), which might lead to a high false positive rate, and might result in unnecessary biopsies and treatment (5). Thus, it is difficult to diagnose and analyze such lesions in clinic, and further exploration is needed.
In recent years, as a supplement to conventional US, contrast-enhanced ultrasound (CEUS) and shear wave elastography (SWE) have provided more diagnostic information. CEUS can give aid to the identification of benign and malignant breast lesions through visualization of the microvascular architecture within the lesion, and is less dependent on operators compared with conventional US (6). The study of Wan et al. (7) showed that the patterns and parameters of CEUS were important for the identification of benign and malignant breast lesions as well as for the prognosis. SWE is the US-based imaging modality, which can depict histologic information by evaluating the mechanical index of tissue (elasticity) (8), so as to differentiate between benign and malignant lesions. SWE has shown potential for differentiating benign from malignant breast disease and could possibly reduce the breast biopsy rate (9-13). Study of Zhou et al. (13) showed that “the stiff rim sign” offered important diagnostic performance for breast lesions, and combination of conventional US and stiff rim sign had the potential to improve the differentiation of breast lesions.
Multi-mode ultrasound is a kind of combination of US, SWE and CEUS, and several studies have shown the diagnostic value of US + CEUS, US + SWE and US + CEUS + SWE for breast lesions (14-16). However, to our knowledge, the comprehensive application of these modalities has not yet formed a unified diagnostic criterion (17). Therefore, the purpose of this study was to investigate the diagnostic value of US, CEUS and SWE and the combination of these modalities for BI-RADS 4 breast lesions and try to figure out a multi-mode ultrasonic method for BIRADS 4 breast lesions.
Methods
Patients
From March 2016 to May 2017, 118 cases of breast lesions diagnosed as BI-RADS 4 lesions by US were included in this study. The age of the patients was 18 to 70 years old (mean age ± standardization, 42.78±10.32 years). The maximal diameter of the lesions ranged from 0.9 to 5.8 cm (mean diameter ± standardization, 1.94±1.0 cm). All the lesions underwent US, CEUS and SWE respectively, and the pathology was confirmed by vacuum-assisted biopsy (n=61) or surgery (n=57).
The inclusion criteria were: (I) patients aged 18 years or older; (II) the lesions were with at least one suspicious sign on US, and were diagnosed as BI-RADS 4 lesions with US. And patients would be excluded if they: (I) lack of CEUS or SWE data; (II) had undergone neoadjuvant chemotherapy previously or were treating with neoadjuvant chemotherapy currently; (III) had undergone radiotherapy previously or were treating with radiotherapy currently; (IV) were pregnant or lactating; (V) lack of pathology results; (VI) had surgery or biopsy previously in the lesion they detected this time; (VII) with breast implants; (VIII) were allergic to sulphur hexafluoride microbubbles (contrast agent); (IX) had severe cardiopulmonary disease. Informed consent was obtained from all patients, and the study was approved by Ethics Committee of Chinese People’s Liberation Army General Hospital. Written informed consent was obtained from every patient at enrollment.
US, CEUS and SWE examinations
US, CEUS and SWE examinations were performed for every lesion. US and CEUS examinations were performed with an IU22 ultrasound system (Philips Medical Systems, Netherlands) with a L12–5 linear array probe, and SWE examinations were performed with Aixplorer ultrasound system (SuperSonic Imagine, Aix en Provence, France) with a probe of 4–12 MHz, and the contrast agent was SonoVue (Bracco, Milan, Italy). All the examinations were performed by two sonographers with more than 10-year experience in ultrasound diagnosis.
The patients were lying in supine or lateral position. Basic characteristics of the lesion (morphology; size; internal echo; margin; calcification; posterior echo; presence of blood flow; aspect ratio and so on) were examined by US and recorded after the examination, and ipsilateral axillary lymph nodes were examined simultaneously.
The plane with the most abundant blood was selected as the CEUS target section, The B-mode pulse inversion harmonic technique was used, and the mechanical index was set at 0.06. The dual-image mode was applied to accurately locate the lesion during the entire CEUS procedure, particularly for tiny lesions. Minimal compression was applied to avoid compressing the vessels. CEUS examination was performed after a bolus injection of 5.0 mL of contrast agent through a cubital vein followed by injection of 5 mL of saline. Real-time dynamic images were recorded for up to 180 s for further analysis.
When SWE was performing, the probe needs to be applied as lightly as possible to give no pressure to the lesion, and kept still for 10–20 s during acquisition of the elastography images (due to a slow frame rate), which was often best done during a breath hold. The elastography views selected were those most clearly displaying abnormal stiffness within the plane without movement or pressure artifacts, and a region of interest (ROI) was chosen to calculate the elasticity value. Because the maximum areas of stiffness in malignant lesions were often found in the peritumoral region rather than in the lesion itself (10), we tried our best to allow ROI to cover the stiffest part of the lesion, rather than to encompass the maximum lesion area. The ROI in the peripheral parenchyma was tried to be of the same size and depth as the ROI in the corresponding breast lesion. For each patient, three ROIs in the lesion and peripheral parenchyma, respectively, were selected, and the mean value was regarded as the final value. In SWE images, the stiff rim sign was defined as increased stiffness (coded in orange or red) in the peritumoral region of the lesion compared with the stiffness in the surrounding breast tissues and the interior lesion tissues. All the data were stored for further analysis.
Image analysis
Basic characteristics of the lesion examined by US were recorded and analyzed by two sonographers with more than 10 years’ experience in ultrasound diagnosis according to the fifth edition of BI-RADS classification standard, finally, the diagnosis result of the lesion was obtained. If disagreement occurred, they would arrive at a consensus by discussion or consulting to a third experienced sonographer.
The enhancement features of the lesions were evaluated according to the literature (18) from the following six aspects: (I) enhancement time: earlier enhancement, later enhancement and synchronous enhancement; (II) distribution of contrast agent: homogeneous, heterogeneous, partial enhancement with perfusion defect and contour enhancement; (III) enhanced intensity: hypo-enhancement, iso-enhancement or hyper-enhancement; (IV) margin of enhanced lesion: clear, less clear or unclear; (V) enhanced area enlargement; (VI) presence of radial or penetrating vessels (crab claw-like enhancement). And the extent of enhancement was greater than 2D image >3 mm as the criteria for enhanced area enlargement (19). Lesions which meet any two or more of these characteristics were classified into BI-RADS 5 and lesions with iso- or hypo-enhancement and clear margin, without enhanced area enlargement and radial or penetrating vessels were classified into BI-RADS 3. Others were classified into 4a, 4b, and 4c according to the difference of characteristics.
The maximum elasticity, mean elasticity, minimum elasticity, the elasticity ratio between lesions and surrounding parenchyma (ratio) and stiff rim sign of the lesion examined by SWE were recorded and the differences among them between the malignant and benign groups were compared.
Then two sonographers diagnosed the lesion considering the US, SWE and CEUS findings and obtain the diagnosis results of multi-mode US. The pathological results obtained by surgery or vacuum-assisted biopsy were taken as the reference and gold standard. And the diagnostic values of conventional US, and the multi-mode US were evaluated, and the cutoff value, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and AUC of each index were analyzed.
Statistical methods
SPSS 19.0 statistical software was used for data analysis. The value of ultrasonic shear wave elasticity was expressed as mean ± SD, χ2 test was used for quantitative data, and t-test was used for qualitative data, P<0.05 was considered statistically significant. The ROC curves were constructed to analyze the diagnostic cutoff, sensitivity, and specificity of CEUS parameters, shear wave elastic parameters, conventional US, and combined evaluation in benign and malignant breast lesions. Logistic regression analysis was used to determine the independent risk factors for benign and malignant lesions.
Results
Pathology
Of the 118 breast BI-RADS 4 lesions, 74 were benign. Of which 45 were fibroadenomas, 11 were intraductal papillomas, 7 were adenopathies, 5 were atypical hyperplasias, 2 were hamartomas, 2 were apocrine adenosis, 1 was phyllodes tumor, and 1 was inflammatory disease. Forty-four were malignant, of which 36 were invasive carcinomas, 7 were ductal carcinomas, and 1 was medullary carcinoma. The pathological results of the 118 breast BI-RADS 4 lesions were shown in Table 1.
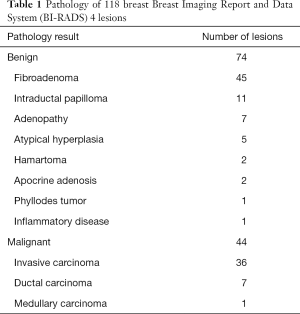
Full table
US characteristics analysis
The US characteristics of the 118 breast BI-RADS 4 lesions could be seen in Table 2. There was significant difference between benign and malignant breast lesions on morphology (P=0.000), margin (P=0.000) and aspect ratio (P=0.000).
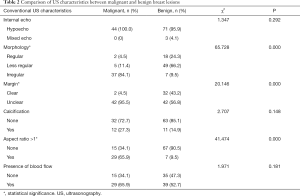
Full table
CEUS enhancement pattern analysis
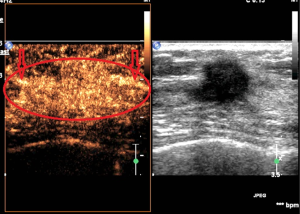
The correlation between pathologic diagnosis and CEUS features of the 118 breast BI-RADS 4 lesions could be seen in Table 3. Benign and malignant breast lesions were significantly different on distribution of contrast agent (P=0.000), enhanced time (P=0.000), enhanced intensity (P=0.000), enhanced area enlargement (P=0.000), presence of radial or penetrating vessels (P=0.000) and margin of enhanced lesion (P=0.000) (Table 3; Figures 1,2).
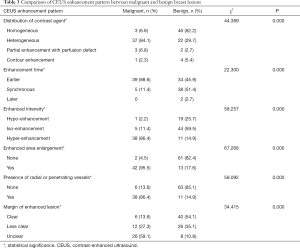
Full table
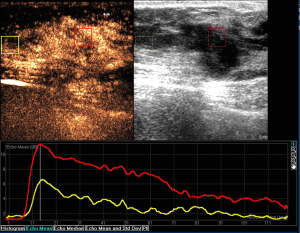
Performance results of SWE features
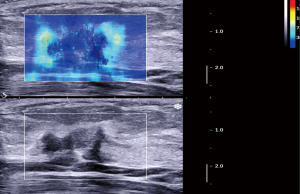
The elasticity parameters of benign and malignant lesions were shown in Table 4. The maximum elasticity, mean elasticity and ratio of the malignant lesions were all significantly higher than those of benign lesions (P=0.000, 0.000, 0.000, respectively), but the minimum elasticity of the malignant lesions was significantly lower than those of benign lesions (P=0.001). When the maximum elasticity 59.0 kPa and ratio 3.25 were set as the diagnostic threshold, the diagnostic sensitivity, specificity, Youden index and accuracy were 79.5% and 81.8%, 98.6% and 93.2%, 0.78 and 0.75, 88.1% and 89.0%, respectively. About 79.5% of the malignant lesions were with a “stiff rim sign” (Figure 3), which was significantly higher than that of benign lesions (6.8%) (P=0.000) (Table 5). And the diagnostic sensitivity, specificity and accuracy of stiff rim sign were 79.5%, 93.2% and 88.1%, respectively. The sensitivity, specificity, Youden index, accuracy of maximum elasticity, mean elasticity, Ratio and stiff rim sign were shown in Table 5.
Full table
Full table
The AUC of ratio [0.897 (95% CI, 0.828–0.946)] was higher than that of stiff rim sign [0.864 (95% CI, 0.789–0.920)], maximum elasticity [0.855 (95% CI, 0.778–0.913)], mean elasticity [0.814 (95% CI, 0.732–0.880)] and minimum elasticity [0.676 (95% CI, 0.584–0.759)]. And there were significant differences between AUC of ratio and mean elasticity (P=0.005), AUC of ratio and minimum elasticity (P<0.0001), AUC of maximum elasticity and minimum elasticity (P=0.0004), AUC of maximum elasticity and mean elasticity (P=0.032), AUC of minimum elasticity and mean elasticity (P=0.003), AUC of minimum elasticity and ratio (P=0.0009). The ROC curve of maximum elasticity, mean elasticity, Ratio and stiff rim sign were shown in Figure 4.
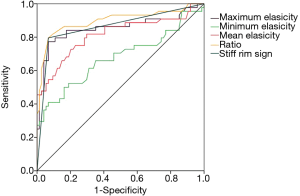
Diagnostic performance of multi-mode US
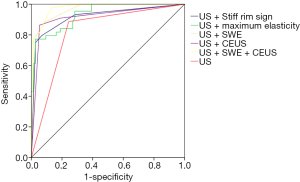
The sensitivity, specificity, PPV, NPV and accuracy of multi-mode US for differentiating benign and malignant breast lesions were outlined in Table 6. Compared with conventional US, US + CEUS, US + SWE and US + SWE + CEUS all significantly improved some of the relevant parameters of the diagnosis of benign and malignant breast lesions. The AUC of US + SWE + CEUS [0.973 (95% CI, 0.926–0.994)] was significantly higher than that of US [0.822 (95% CI, 0.740–0.886)] (P<0.0001), US + CEUS [0.918 (95% CI, 0.853–0.960)] (P=0.020), US + maximum elasticity [0.934 (95% CI, 0.873–0.971)] (P=0.028) and US + stiff rim sign [0.920 (95% CI, 0.856–0.962)] (P=0.032). And the AUC of US + SWE + CEUS was also higher than that of US + SWE [0.965 (95% CI, 0.914–0.990)], but there was no statistical significance (P=0.263) (Figure 5). Thus, US + SWE + CEUS and US + SWE had better diagnostic efficiency.

Full table
False positive diagnosis with conventional US combined with SWE and CEUS
The false positive rate was 6.8% (5/74) when the combination of conventional US, SWE and CEUS was applied to the differential diagnosis of benign and malignant lesions. The false positive breast lesions were sclerosing adenosis (n=3), adenopathy (n=1) and chronic inflammatory disease (n=1), respectively.
Single factor analysis of the indicators for malignant lesions
As shown in Tables 2-5, the single factor analysis showed that factors related to the differential diagnosis of benign and malignant breast lesions included morphology, margin and aspect ratio on conventional US; distribution of contrast agent, enhancement time, enhanced lesion morphology, enhanced area enlargement, presence of radial or penetrating vessels and margin of enhanced lesion on CEUS; maximum elasticity, mean elasticity, minimum elasticity, ratio and stiff rim sign on SWE. In other words, all of these factors were closely related to the characteristics of malignant breast lesions according to the results of single factor analysis.
Results of multivariable logistic regression analysis
A multivariate logistic regression analysis was needed to exclude the effect of confounding factors in single factor analysis and to explore which factors were related to the differential diagnosis of benign and malignant breast lesions. All the features obtained from single factor analysis were included in the regression analysis as independent variables. The pathology results were used in the regression analysis as dependent variables (0= benign, 1= malignant) and then established dummy variables. Next, the multivariable logistic regression analysis was performed and the final identified independent risk factors were less regular morphology, irregular morphology, and aspect ratio >1 on US, enhanced area enlargement on CEUS and ratio on SWE, all of which were statistically significant (P=0.024, 0.032, 0.044, 0.042, 0.032, respectively). For convenience, a simple multi-mode method was established based on the logistic regression formula, and the final logistic regression equation was as follows:
LogitP =3.898 X1 − 10.583 X2 − 13.656 X3 − 16.791 X4 + 4.857 X5
The variable X1 represented ratio; X2 represented enhanced area enlargement; X3 represented less regular morphology on US; X4 represented irregular morphology on US; X5 represented aspect ratio >1.
Then based on pathological diagnosis as the golden criterion, the ROC curve was plotted for this formula, and the AUC was 0.990. When the cutoff point was 0.290 according to the Youden index, the sensitivity, specificity, PPV, NPV and accuracy were 0.977, 0.954, 0.915, 0.986 and 0.958, respectively.
Discussion
According to the 5th edition of the ACR BI-RADS-US lexicon (1), BI-RADS 4 lesions hold a broad range of malignant risk of 3–94%. And in our study, 62.7% of the 118 lesions diagnosed as BI-RADS 4 by conventional US were pathologically demonstrated to be benign. Thus, new US technologies are needed to further clarify the nature of lesions, so as to discover malignant lesions as early as possible and to avoid unnecessary biopsies of benign lesions. Both CEUS and SWE could provide extra information about lesions beyond morphology, such as the micro-circulation of tumor and the elasticity of tissue, etc., which will make up for the deficiency of conventional US, and the diagnostic value of both methods for benign and malignant breast lesions had been confirmed (20,21).
Our research showed that lesions with irregular morphology, unclear margin and aspect ratio >1 were tend to be malignant, which was consistent with previous studies (2). Thus, we would have irregular morphology, unclear margin, and aspect ratio >1 defined as powerful parameters for the diagnosis of malignant breast lesions.
Our research showed that most of the benign breast lesions (85.2%) showed iso-enhancement or hypo-enhancement on CEUS. In contrast, the perfusion patterns of most of the malignant lesions were characterized by hyper-enhancement (86.4%). These might be caused by the tumor angiogenesis factor produced by the breast tumor cells, which could act on the malignant vascularity (22). Thus, the malignant vascularity would grow very easily and fast and characterize by thin wall, small lumen, incomplete endothelium and without smooth muscle cell and nerve terminal, which was significantly different from the normal and benign vascularity (22). Therefore, the malignant tumor vascularity would lack systolic and diastolic function on account of the above-mentioned features, which could result in a high blood perfusion (22). Thus, malignant breast lesions were mostly manifested as hyper-enhancement. Conversely, benign lesions vessels were less affected by tumor angiogenesis factor, so they appeared to be characterized by slow growth, low blood perfusion, complete endothelium and with smooth muscle cell and nerve terminal, which might result in the perfusion patterns of iso-enhancement or hypo-enhancement of benign breast lesions.
Tumor angiogenesis factor also could act on the surrounding normal tissue, especially endothelial cells of the surrounding blood vessels, which might result in the appearance of radial or penetrating vessels (23), and then explain the appearance of crab claw-like enhancement in most malignant breast lesions (86.4%). The study of Wan et al. (7) showed that the presence of radial or penetrating vessels after enhancement was highly correlated with malignancy with a diagnostic specificity of 97.7%, which was higher than that of our study (85.1%). This difference might be closely related to the differences in the instrument used and in the operator’s definition of radial or penetrating blood vessels and so on.
In our study, 95.5% of the malignant lesions showed enhanced area enlargement, which was significantly higher than that of benign lesions (17.6%). Thus, we defined it as an important characteristic for the identification of malignant lesions. The study of Aitken et al. (24) showed that enhanced area enlargement was a sign of malignancy. And the study of Zhao et al. (25) told us that enhanced area enlargement revealed that the breast lesions were rich in angiogenesis. We would suggest that, tumor angiogenesis factors could act on malignant vascularity, causing malignant vascularity to grow very easily and quickly, which in turn makes malignant lesions rich in angiogenesis, resulting in enhanced area enlargement.
Our study showed a significant difference in the distribution of contrast agents between benign and malignant breast lesions (P=0.000). The majority of malignant lesions (84.1%) manifested as heterogeneous enhancement, while benign lesions mostly showed homogeneous enhancement (62.2%). According to the study of Metz et al. (26), malignant lesions grew faster than did benign lesions, which might result in insufficient vascular formation and nutrition supply. Thus, some parts of the tumor might become hypoxic and necrotic, the distribution of the internal microvessels of the lesion might also have changed, resulting in the occurrence of perfusion defects and heterogeneous enhancement. Buadu et al. (27) reported that the distribution of microvessel played a major role in the heterogeneous enhancement of the malignant lesions. Thus, in our study, the findings of perfusion defects or heterogeneous enhancement were observed in 90.9% of the malignant lesions can be explained by the internal necrosis of the lesions and the heterogeneous distribution of the vascularity.
Stiffness was another important factor for the differential diagnosis for benign and malignant lesions. Previous studies had indicated that the elasticity of benign and malignant breast lesions was closely related with the tissue stiffness of breast lesions (28) and SWE could provide quantitative elasticity information that can potentially help characterize breast lesions (12). This study showed that the maximum elasticity, mean elasticity, and ratio of malignant lesions were all significantly higher than those of benign lesions, but the minimum elasticity of the malignant lesions were significantly lower than those of benign lesions, which was not consistent with our previous study (28), and the difference may be related to the difference in the composition of the lesions in the two studies and the difference in the operator during the SWE examination. We also found that when 59 kPa was set as the cutoff value of the maximum elasticity, the specificity was significantly higher than that of conventional US. And when 3.25 was set as the cutoff value of ratio, the specificity and accuracy were significantly higher than that of conventional US. It demonstrated that max elasticity >59 kPa and ratio >3.25 might be differential diagnostic indicators for benign and malignant breast lesions.
Also, we found in this study that the incidence of stiff rim sign in malignant lesions (79.5%) was significantly higher than that in benign lesion (6.8%) (P=0.000), and compared with conventional US, stiff rim sign showed higher specificity (93.2%) and accuracy (88.1%) and a lower sensitivity (79.5%), which was somewhat different from the results of a previous study (13). A study of Zhou et al. (13) showed that compared with conventional US, stiff rim sign showed higher sensitivity and specificity, this difference might be due to the differences in the types of diseases included in the sample and the differences in the aspects selected for SWE. Previous studies (10,29) suggested that the stiff rim sign might be caused by the attenuation of shear wave energy caused by the proliferative reaction to connective tissue or the infiltration of cancer cells into the peripheral tissues. Thus, stiff rim sign would be more common in malignant breast lesions.
In this study, the logistic regression model with the combination of conventional US, SWE and CEUS was used for the differential diagnosis of benign and malignant breast lesions. As described above, the study showed that breast lesions with less regular or irregular morphology, aspect ratio >1, heterogeneous enhancement, enhanced area enlargement, crab-claw like enhancement, maximum elasticity >59 kPa, ratio >3.25 and with stiff rim sign and so on were more likely to be malignant. Thus, we defined all of these features as risk factors, and put them into logistic regression analysis. But only less regular morphology, irregular morphology and aspect ratio >1 on US, ratio on SWE and enhanced area enlargement on CEUS were included in the equation. Therefore, with less regular morphology, irregular morphology and aspect ratio >1 on US, ratio on SWE and enhanced area enlargement on CEUS as the independent risk factors, it might provide sonographers a new multi-mode modality for the differential diagnosis of BI-RADS 4 category lesions, which was beneficial for developing follow-up treatment plans for clinical patients.
The multi-mode US method developed on the logistic regression formula was simple with a high diagnostic efficiency. US provided the information about the basic characteristics of the lesions, SWE provided the information about elasticity and CEUS provided information about the blood supply, the combination of the three could produce a more intuitive and accurate understanding of the lesions. We found that the combination of conventional US, SWE and CEUS improved the performance of conventional US in identifying benign and malignant breast lesions, with a significantly increase in AUC from 0.822 to 0.973 (P<0.0001), and also in specificity, PPV and accuracy. We also found that compared with US, US + CEUS, US + maximum elasticity and US + stiff rim sign, US + SWE + CEUS showed the best diagnostic efficiency, but without statistical difference from US+SWE. This was different from the results of Xiao et al.’s study (30) which showed that the diagnostic efficiency of US + CEUS was the best. The reason might be that the study of Xiao et al. (30) only focused on breast lesions <1 cm in diameter whereas our study didn’t take the diameter of the lesions into account.
Of the 118 BI-RADS 4 breast lesions, 5 cases were over-estimated. Of which, 3 were sclerosing adenosis, 1 was adenopathy and 1 was chronic inflammatory disease. Fibrocystic changes in sclerosing adenosis could be hard on SWE, which might result in the “stiff rim sign” and ratio >3.25. And chronic mastitis could show heterogeneous hyperenhancement and enhanced area enlargement on CUES due to the infiltration of inflammatory cells. And adenopathy might be hard on SWE because of fibrous hyperplasia and showed a same enhancement pattern. These ultrasonographic findings are similar to malignancies, which led to the misdiagnosis.
The study has several limitations. First, the ROI was circular, thus it was difficult to cover the entire lesion, especially for lesions with irregular borders. Second, the features of conventional US such as posterior echo, size, location and so on were not analyzed by single factor analysis and multivariable logistic regression analysis, which might lead to bias.
In conclusion, the combination of conventional US, CEUS and SWE and US + SWE could improve the diagnostic efficiency and accuracy greatly for the differential diagnosis of breast BI-RADS 4 lesions. Although the diagnostic efficacy of US + CEUS + SWE is not significantly higher than that of US + SWE, CEUS examination could help to analyze the microscopic perfusion of the lesion, thereby providing a deeper understanding of the features of the lesion and providing help for subsequent core-needle biopsy or vacuum-assisted biopsy of the lesion. Therefore, when diagnosing breast lesions, we should choose US + CEUS + SWE. And, it might be helpful for predicting the potential malignant breast lesions.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation (81771832); the Capital characteristics of the fund (Z161100000516190); and the Military health issues (17BJZ34).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Informed consent was obtained from all patients, and the study was approved by Ethics Committee of Chinese People’s Liberation Army General Hospital. Written informed consent was obtained from every patient at enrollment.
References
- Spak DA, Plaxco JS, Santiago L, et al. BI-RADS((R)) fifth edition: A summary of changes. Diagn Interv Imaging 2017;98:179-90.
- Elverici E, Barca AN, Aktas H, et al. Nonpalpable BI-RADS 4 breast lesions: sonographic findings and pathology correlation. Diagn Interv Radiol 2015;21:189-94. [Crossref] [PubMed]
- Heinig J, Witteler R, Schmitz R, et al. Accuracy of classification of breast ultrasound findings based on criteria used for BI-RADS. Ultrasound Obstet Gynecol 2008;32:573-8. [Crossref] [PubMed]
- Raza S, Chikarmane SA, Neilsen SS, et al. BI-RADS 3, 4, and 5 lesions: value of US in management--follow-up and outcome. Radiology 2008;248:773-81. [Crossref] [PubMed]
- Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev 2013.Cd009632. [PubMed]
- Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33-59. [Crossref] [PubMed]
- Wan CF, Du J, Fang H, et al. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology 2012;262:450-9. [Crossref] [PubMed]
- Barr RG, Nakashima K, Amy D, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: breast. Ultrasound Med Biol 2015;41:1148-60. [Crossref] [PubMed]
- Amano M, Ogura K, Ozaki Y, et al. Two cases of primary small cell carcinoma of the breast showing non-mass-like pattern on diagnostic imaging and histopathology. Breast Cancer 2015;22:437-41. [Crossref] [PubMed]
- Evans A, Whelehan P, Thomson K, et al. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res 2010;12:R104. [Crossref] [PubMed]
- Sotome K, Yamamoto Y, Hirano A, et al. The role of contrast enhanced MRI in the diagnosis of non-mass image-forming lesions on breast ultrasonography. Breast Cancer 2007;14:371-80. [Crossref] [PubMed]
- Wang ZL, Li JL, Li M, et al. Study of quantitative elastography with supersonic shear imaging in the diagnosis of breast tumours. Radiol Med 2013;118:583-90. [Crossref] [PubMed]
- Zhou J, Zhan W, Chang C, et al. Breast lesions: evaluation with shear wave elastography, with special emphasis on the "stiff rim" sign. Radiology 2014;272:63-72. [Crossref] [PubMed]
- Leng X, Huang G, Yao L, et al. Role of multi-mode ultrasound in the diagnosis of level 4 BI-RADS breast lesions and Logistic regression model. Int J Clin Exp Med 2015;8:15889-99. [PubMed]
- Li X, Li Y, Zhu Y, et al. Association between enhancement patterns and parameters of contrast-enhanced ultrasound and microvessel distribution in breast cancer. Oncol Lett 2018;15:5643-9. [PubMed]
- Ren WW, Li XL, Wang D, et al. Evaluation of shear wave elastography for differential diagnosis of breast lesions: A new qualitative analysis versus conventional quantitative analysis. Clin Hemorheol Microcirc 2018;69:425-36. [Crossref] [PubMed]
- Opielinski KJ, Pruchnicki P, Gudra T, et al. Imaging results of multi-modal ultrasound computerized tomography system designed for breast diagnosis. Comput Med Imaging Graph 2015;46:83-94. [Crossref] [PubMed]
- Du J, Li FH, Fang H, et al. Correlation of real-time gray scale contrast-enhanced ultrasonography with microvessel density and vascular endothelial growth factor expression for assessment of angiogenesis in breast lesions. J Ultrasound Med 2008;27:821-31. [Crossref] [PubMed]
- Zeggelink WF, Deurloo EE, Bartelink H, et al. Reproducibility of the assessment of tumor extent in the breast using multiple image modalities. Med Phys 2003;30:2919-26. [Crossref] [PubMed]
- Zhang JX, Cai LS, Chen L, et al. CEUS helps to rerate small breast tumors of BI-RADS category 3 and category 4. Biomed Res Int 2014;2014:572532. [PubMed]
- Zhao YX, Liu S, Hu YB, et al. Diagnostic and prognostic values of contrast-enhanced ultrasound in breast cancer: a retrospective study. Onco Targets Ther 2017;10:1123-9. [Crossref] [PubMed]
- Zhao H, Xu R, Ouyang Q, et al. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur J Radiol 2010;73:288-93. [Crossref] [PubMed]
- Lichtenbeld HC, Barendsz-Janson AF, van Essen H, et al. Angiogenic potential of malignant and non-malignant human breast tissues in an in vivo angiogenesis model. Int J Cancer 1998;77:455-9. [Crossref] [PubMed]
- Aitken E, Osman M. Factors affecting nodal status in invasive breast cancer: a retrospective analysis of 623 patients. Breast J 2010;16:271-8. [Crossref] [PubMed]
- Zhao LX, Liu H, Wei Q, et al. Contrast-Enhanced Ultrasonography Features of Breast Malignancies with Different Sizes: Correlation with Prognostic Factors. Biomed Res Int 2015;2015:613831. [Crossref] [PubMed]
- Metz S, Daldrup-Unk HE, Richter T, et al. Detection and quantification of breast tumor necrosis with MR imaging: value of the necrosis-avid contrast agent Gadophrin-3. Acad Radiol 2003;10:484-90. [Crossref] [PubMed]
- Buadu LD, Murakami J, Murayama S, et al. Patterns of peripheral enhancement in breast masses: correlation of findings on contrast medium enhanced MRI with histologic features and tumor angiogenesis. J Comput Assist Tomogr 1997;21:421-30. [Crossref] [PubMed]
- Wang ZL, Sun L, Li Y, et al. Relationship between elasticity and collagen fiber content in breast disease: a preliminary report. Ultrasonics 2015;57:44-9. [Crossref] [PubMed]
- Barr RG. Shear wave imaging of the breast: still on the learning curve. J Ultrasound Med 2012;31:347-50. [Crossref] [PubMed]
- Xiao X, Jiang Q, Wu H, et al. Diagnosis of sub-centimetre breast lesions: combining BI-RADS-US with strain elastography and contrast-enhanced ultrasound-a preliminary study in China. Eur Radiol 2017;27:2443-50. [Crossref] [PubMed]


