Minimizing incisional dehiscence following 2-stage prosthetic breast reconstruction in the setting of radiation therapy
Introduction
Minimizing morbidity in the setting prosthetic breast reconstruction associated with pre or post mastectomy radiation therapy (RT) remains an important area of clinical research. Studies have demonstrated that complications leading to prosthetic failure are increased in the setting of RT compared to the non-radiated breast and ranges from 40-45% (1,2). Some of these untoward events include capsular contracture, infection, device exposure, and cutaneous fibrosis. Etiology is multifactorial; however, damage to the subdermal vascular plexus, subcutaneous fat atrophy, cutaneous fibrosis, and skin tension are usually implicated.
A particular topic that has become increasingly appreciated is prosthetic exposure due to incisional dehiscence following the second stage of reconstruction in the setting of prior RT. Studies have demonstrated that the incidence of incisional dehiscence ranges from 10-15% in the setting of RT compared to 1-2% without RT (1,2). This observation has been noted when the incision to exchange the tissue expander for a permanent implant is made through the original mastectomy scar.
The purpose of this study is to describe a technical modification that can minimize the incidence of incisional dehiscence during the second stage of prosthetic reconstruction in the setting of previous RT.
Methods/description of technique
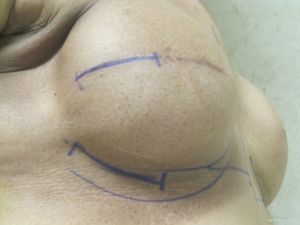
At the time of exchange for the tissue expander to a permanent implant, the surgeon has two options to access the periprosthetic pocket. The first is to go through the original scar and the second is to create an incision at a new site (Figure 1). The modification utilized involves creating a new incision along the infero-lateral aspect of the inframammary fold (IMF).
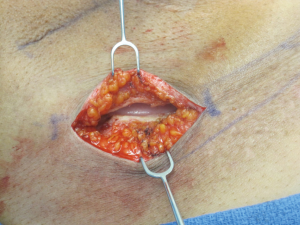
Preoperatively, with the patient in the standing position, the IMF is delineated. The midline of the IMF is marked and is extended laterally for a distance that ranges from 4-6 centimeters depending on the volume of the device being removed as well as being inserted. Following the skin incision, the dissection extends through the subcutaneous tissue until the capsule is identified. The capsule is incised and the periprosthetic space is entered (Figure 2). The tissue expander is removed either intact or surgically deflated. Using a lighted retractor, headlamp, or overhead lighting, a capsulotomy or capsulectomy can be performed depending on the severity of capsule formation. The space is irrigated using an antibacterial solution, a closed suction drain is usually inserted, the skin is prepped again with a povidone-iodine solution, and the permanent implant is inserted. The incision is closed in four layers that include the capsule, subcutaneous fat, dermis and epidermis. The epidermis can be approximated with an absorbable subcuticular suture or using a nonabsorbable interrupted vertical mattress suture. These sutures are usually removed 2-3 weeks following the operation.
Results
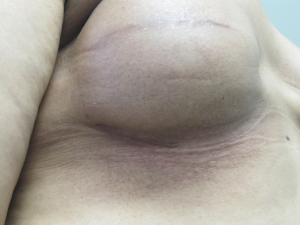
This technique has been used in 29 patients with tissue expanders that have had radiation either before skin sparing mastectomy (n=6) or after skin sparing mastectomy (n=23). No patients were noted to develop skin necrosis or delayed healing. Adverse events have occurred in 2 patients (6.9%). In the first patient, incisional dehiscence occurred in the setting of a previous IMF incision that was in the field of RT. There was no evidence of infection and this was felt to represent a mechanical problem related to the soft tissues. In this patient, the skin was debrided, the periprosthetic space was copiously irrigated with an antimicrobial solution, a closed suction drain was inserted, the device was exchanged for a smaller implant, and a layered closure was performed. In the second patient there was drainage noted from the incision due to a periprosthetic infection. In this patient the device was removed, the skin was debrided, the space was copiously irrigated with an antimicrobial solution, a closed suction drain was placed, and a layered closure was performed. Future implant reconstruction was not recommended and the patient underwent a successful reconstruction using autologous tissue. Long-term outcomes have been excellent 27/29 breasts (94%, Figure 3).
Discussion
In patients who have had a skin sparing mastectomy, tissue expander reconstruction, and RT, there are several noteworthy observations. In most cases, the incision is at the apex of the breast and has been directly targeted by the radiation. In some patients, an additional boost of electrons is delivered specifically to the incision site to enhance the tumoricidal effect. The effects on the targeted soft tissues typically include subcutaneous thinning or atrophy that is a consequence of RT as well as from the overlying pressure exerted by the expanding device.
Most plastic surgeons will typically exchange the tissue expander for a permanent implant following RT by re-excising the prior mastectomy scar. This is followed by a “step-ladder” approach through the soft tissues such that the cutaneous and the capsular incision are off-set. In our previous study in whom incisional dehiscence occurred, the cutaneous structures were noticed to be very thin with a paucity of subcutaneous fat (1). The closure typically consisted of 2 layers of absorbable suture placed in the capsule/dermis followed by a subcuticular suture in the epidermis. Of those patients that experienced a dehiscence, it usually occurred 3-4 weeks postoperatively with a common theme that they were reaching for something when they felt the dehiscence.
There are clinical studies that confirm the fact that entering a breast implant pocket through a previously radiated incision will increase the likelihood of incisional dehiscence. In one study comparing non-radiated to radiated prosthetic reconstruction, Nahabedian demonstrated incisional dehiscence in 1/77 (1.3%) breasts that were not radiated compared to 3/23 (13%) that were radiated (1). All dehiscence’s occurred following the conversion of the tissue expander to the permanent implant. In another study, Nava demonstrated that device exposure due to incisional dehiscence was increased when RT was delivered prior to device exchange (7/50, 14%) compared to following device exchange (1/109, 0.9%) (2). There was good concordance between these two studies.
Based on these findings, it can be extrapolated that the exchange of a tissue expander for a permanent implant should ideally occur before RT. This has been the approach advocated by the Memorial Sloan Kettering (3). A caveat to this approach is that there must be enough time between the mastectomy and the radiation. Typically RT is commenced 3-4 weeks following mastectomy unless patients receive chemotherapy. This leaves little time to achieve optimal expansion. As a result, most surgeons tend to perform the exchange following RT.
Thus, in order to minimize the incidence of incisional dehiscence, the infero-lateral IMF counter-incision has been routinely performed in the setting of prosthetic reconstruction and RT. This approach has been used in 29 patients with only 1 true dehiscence noted that occurred in a patient that had a prior inframammary incision. This confirmed that re-entering a previously radiated scar is prone to incisional dehiscence based on mechanical factors. Obviously, infection can be another cause of incisional failure with or without radiation.
Initial concerns utilizing the IMF counter incision were that delayed healing may occur because of the bipedicle nature of the prior mastectomy incision and the new IMF incision. This has not been the case as no patients have experienced delayed healing or tissue necrosis. This is most likely because of the vascular delay effect and the vascularity of the capsule. Reasons for the success of this approach, despite being within the radiated field, include a subcutaneous layer of normal or reasonable thickness and the ability to close the incision in 3-4 layers that includes the capsule, subcutaneous tissue, dermis, and epidermis. Another reasons is that the IMF is not an area that typically receives a boost of electrons so the vascularity and tissue quality may be less compromised.
In summary, this series of patients illustrates that a infero-laterally based incision during the second stage of prosthetic reconstruction can reduce complications related to incisional dehiscence. Morbidities related to the incision can still occur but have been related to extenuating circumstances that include a previously radiated scar and infection. This approach is currently being performed by the author for nearly all patients that have had previous RT.
Acknowledgements
Disclosure: Dr. Nahabedian is a speaker and consultant for LifeCell Corporation. No financial or administrative assistance was obtained in preparing this manuscript.
References
- Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg 2009;124:1743-53. [PubMed]
- Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg 2011;128:353-9. [PubMed]
- Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer 2012;118:2552-9. [PubMed]