
Radioisotope imaging for discriminating benign from malignant cytologically indeterminate thyroid nodules
Introduction
The evaluation of thyroid nodules includes measurement of serum thyroid stimulating hormone (TSH), ultrasound examination, and ultrasound-guided fine-needle cytology (1). In addition, thyroid scintigraphy can be performed to assess the function of thyroid nodules. Hyperfunctioning thyroid nodules have a very low incidence of malignancy and may be treated without undergoing fine-needle cytology. Non-hyperfunctioning thyroid nodules have a higher incidence of malignancy and fine-needle cytology may be required depending on morphological criteria from ultrasound imaging (2).
When cytological results yield follicular lesion or atypia of undetermined significance (Bethesda III) or follicular neoplasm (Bethesda IV), the results are considered to be indeterminate. The risk of malignancy in thyroid nodules with indeterminate cytological classification ranges from 10% to 40%, and early delineation is essential as delays in diagnosis can be associated with increased mortality (3,4). However, the majority of patients with nodules of indeterminate cytological characteristics undergoing thyroid resection are ultimately found to have a benign lesion. Therefore, therapeutic decision-making in patients with thyroid nodules with indeterminate cytology can be challenging.
Several radioisotope imaging techniques are available for discriminating benign from malignant cytologically indeterminate thyroid nodules and for supporting clinical decision-making. This review discusses the currently available radioisotope imaging techniques for evaluation of thyroid nodules, including the mechanism of radiotracer uptake and the indications for their use.
Iodine-123 and technetium-99m-pertechnetate
Iodine-123 physiology
Thyroid follicular cells trap iodine using the sodium-iodine-symporter, which concentrates iodine intracellularly at 25–500 times the plasma concentration, and incorporate the iodine into thyroglobulin (5-7). Therefore, iodine is oxidized by thyroid peroxidase at the follicular cell colloid interface to neutral iodine. The neutral iodine then binds to tyrosine residues on the thyroglobulin, where it is incorporated into thyroid hormone and stored in the colloid follicular lumen. Thyroid hormones can then be released from thyroglobulin into the blood stream when necessary (Figure 1).
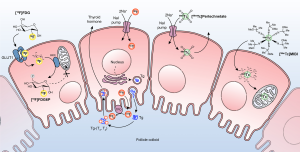
The gamma-emitter iodine-123 is an iodine isotope, and thus is metabolized identically to naturally occurring non-radioactive iodine. It is taken up by thyroid follicular cells via the sodium-iodine-symporter, then is incorporated into thyroglobulin and stored in the thyroid follicles. During this process, the gamma emission of iodine-123 allows for imaging via scintigraphy for several hours after injection.
Iodine-123 scintigraphy is generally used for diagnosis of thyroid diseases, including hyperthyroidism and congenital hypothyroidism, as well as for the evaluation of nodules. In addition, it is used in the post-operative setting and for follow-up of differentiated thyroid cancers as an alternative to iodine-131 (2).
Technetium-99m-pertechnetate physiology
The gamma-emitter technetium-99m-pertechnetate is taken up by thyroid follicular cells in the same manner as iodine via the sodium-iodine-symporter. However, contrary to iodine, it is not incorporated into thyroglobulin (Figure 1). Thus, it is not retained in the thyroid tissue and scintigraphy must be performed 20–30 minutes after tracer injection. Technetium-99m-pertechnetate scintigraphy is generally used for assessing hyperthyroidism and thyroid nodules.
Iodine-123 and technetium-99m-pertechnetate in cytologically indeterminate thyroid nodules
Thyroid scintigraphy with either iodine-123 or technetium-99m-pertechnetate reflects the metabolic rate of thyroid cells, and tracer uptake therefore demonstrates the presence of functioning thyroid tissue (8). As it is highly uncommon for autonomously functioning thyroid nodules, also known as “hot nodules”, to harbor malignancy (9-11), current clinical guidelines recommend abstaining from performing fine-needle biopsy of hyperactive nodules diagnosed by thyroid scan with either iodine-123 or technetium-99m-pertechnetate (2,12,13). A further reason underlying this recommendation is that a certain number of fine-needle cytologies in autonomously functioning nodules may be diagnosed as suspicious for a follicular neoplasm (Bethesda IV), which would then result in an increased number of unnecessary lobectomies or thyroidectomies in benign lesions.
The 2015 guideline of the American Thyroid Association (ATA) recommends a thyroid scan, preferably with iodine-123, only in the case of low or subnormal TSH (2). The guideline of the European Association of Nuclear Medicine (EANM), however, declined to endorse this recommendation (14). The EANM Thyroid Committee and several independent authors support that thyroid scintigraphy still has its place in the diagnostic work-up of nodular disease, particularly in regions with a known alleviated iodine deficiency, and that a normal TSH level does not preclude the need for thyroid scintigraphy (14,15). In such populations, a normal TSH level cannot rule out thyroid autonomy, as recommended by the EANM Thyroid Committee (14,16-19).
The use of thyroid scintigraphy to select “cold” nodules for a cytological analysis significantly reduces the number of surgical procedures for benign diseases, particularly in populations with iodine deficiency-related nodular disease (20). In certain cases, the uptake of a thyroid nodule can be discordant between iodine-123 and technetium-99m-pertechnetate, and thus some guidelines, including the ATA guideline, recommend using iodine-123 for the functional evaluation of thyroid nodules (2,12) as opposed to technetium-99m-pertechnetate. As up to 5% of thyroid nodules may demonstrate rapid washout linked to a defect in the process of integrating iodine into thyroglobulin, visualization of these nodules requires both early (1–4 hours) and late (24 hours) imaging with iodine-123 (21) and is therefore not feasible with technetium-99m-pertechnetate.
Technetium-99m-methoxy-isobutyl-isonitrile (technetium-99m-MIBI)
Technetium-99m-MIBI physiology
Technetium-99m-MIBI is a lipophilic monovalent cationic agent from the family of isonitriles, which concentrates in the mitochondria due to its electric potential (Figure 1). In a thyroid nodule, technetium-99m-MIBI uptake reflects actively functioning mitochondria, and therefore cellular oxidative metabolism (16).
Technetium-99m-MIBI in cytologically indeterminate thyroid nodules
A meta-analysis of 21 studies showed that technetium-99m-MIBI scintigraphy is a sensitive diagnostic tool in predicting malignancy when malignancy is suspected on the basis of ultrasound. In a per-lesion analysis, the pooled sensitivity and specificity were 85% and 46%, respectively (22). A higher diagnostic accuracy can be achieved when only “cold” nodules are considered, based on previous iodine-123 or technetium-99m-pertechnetate scintigraphy, with a pooled sensitivity and specificity of 82% and 63%, respectively (10,22). Therefore, combining technetium-99m-MIBI scintigraphy with iodine-123 or technetium-99m-pertechnetate scintigraphy reduces the risk of false positive results (23,24). A recent prospective study found a negative predictive value of 100% to rule out malignant thyroid nodules, if nodules presented “cold” at technetium-99m-pertechnetate scintigraphy and “negative” at technetium-99m-MIBI scintigraphy (25).
The sensitivity, specificity, accuracy, positive predictive value and negative predictive value of technetium-99m-MIBI scintigraphy were 100%, 90%, 95%, 88% and 100%, respectively, in differentiating benign from malignant non-oncocytic thyroid lesions when the interpretation was based on semi-quantitative analysis (23). Moreover, the diagnostic accuracy of technetium-99m-MIBI scintigraphy can be increased by tomographic 3D acquisition, in addition to planar images.
The results are more heterogeneous concerning the ability to discriminate benign from malignant oncocytic thyroid lesions, and there is no recommendation to use technetium-99m-MIBI in these lesions (25-27). Retention of technetium-99m-MIBI uptake in benign thyroid nodules may occur in hyperplasic nodular goiter, macro and micro-follicular adenoma, Hürthle cell adenoma, or autoimmune or sub-acute nodular thyroiditis (8). Consequently, a positive technetium-99m-MIBI scan should be regarded as "indeterminate".
Several studies have evaluated the diagnostic performance of technetium-99m-MIBI scintigraphy in the specific setting of patients with cytological indeterminate thyroid nodules. One study, for example, reported a sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of technetium-99m-MIBI visual image analysis in differentiating benign from malignant non-oncocytic tumors of 73%, 81%, 73%, 81% and 78%, whereas the performance for oncocytic tumors was 100%, 9%, 28%, 100% and 33%. Notably, in non-oncocytic thyroid nodules, a technetium-99m-MIBI semi-quantitative image analysis was more accurate than a technetium-99m-MIBI visual image analysis with a diagnostic accuracy of 94% vs. 78% (26).
A more recent prospective study reported a sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of visual analysis of technetium-99m-MIBI scintigraphy in differentiating benign from malignant cytologically indeterminate thyroid nodules of 56%, 52%, 23%, 82%, and 53% (28). Another prospective study demonstrated a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of technetium-99m-MIBI scintigraphy in differentiating benign from malignant disease of 62%, 95%, 83%, 88%, and 87% in patients with hypofunctioning thyroid nodules on technetium-99m-pertechnetate scintigraphy, findings suggestive of malignancy on ultrasound examination, and cytological classification as a follicular neoplasm (Bethesda IV). By using semi-quantitative analysis, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy increased to 100%, 96%, 88%, 100%, and 98% (25).
Finally, a recent bi-centric study demonstrated a sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of visual technetium-99m-MIBI analysis in differentiating malignant from benign cytologically indeterminate thyroid nodules of 61%, 91%, 71%, 86%, and 83%. In the semi-quantitative technetium-99m-MIBI analysis, the overall sensitivity, specificity, positive predictive value, negative predictive value, and accuracy increased to 100%, 91%, 80%, 100%, and 93% (29).
Various methods of semi-quantitative methods for the technetium-99m-MIBI analysis have been proposed (25,26,30). The implementation of wash-out index was recently found to be more accurate than the retention index in discriminating malignant from benign cytological indeterminate thyroid nodules with an overall accuracy of 100% vs. 62.5%, respectively (31).
Overall, technetium-99m-MIBI scintigraphy with additional semi-quantitative analysis seems to be an accurate and cost-effective method for evaluation of cytological indeterminate thyroid nodule (32). However, there is no recommendation for the use of technetium-99m-MIBI scintigraphy in the evaluation of thyroid nodules in the current guidelines (2).
Fluorine-18-fluorodeoxyglucose (Fluorine-18-FDG)
Fluorine-18-FDG physiology
Fluorine-18-FDG is an analogue of glucose and is transported into the cell by transmembrane glucose transporters in the same manner as naturally-occurring glucose molecules (33). The intracellular fluorine-18-FDG is then phosphorylated by hexokinase to fluorine-18-FDG-6-phosphate, which traps the compound and prevents its efflux (Figure 1). In malignant cells, both the expression of GLUT1 transporters and the activity of hexokinase may be increased (34). Unlike naturally-occurring glucose molecules, fluorine-18-FDG-6-phosphate cannot proceed down the glycolytic pathway and therefore accumulates in cancerous cells.
Indications in the diagnosis of thyroid nodules and guideline recommendations
Fluorine-18-FDG positron emission tomography (PET)-computed tomography (CT) is an important diagnostic modality for various cancers (35). The ATA guidelines, however, do not recommend fluorine-18-FDG PET for the evaluation of thyroid nodules (36). This recommendation is based on two meta-analyses where the pooled sensitivity and specificity of fluorine-18-FDG PET in indeterminate thyroid nodules based on fine needle cytology were 95% and 48% respectively, resulting in a positive predictive value of 39% and a negative predictive value of 96% (37,38). Thereby, the common practice is a visual assessment that considers any focal increased uptake in the region of the thyroid nodule above the background as positive (37). To investigate if a quantitative approach will improve the diagnostic accuracy remains preserved for future research.
It has been suggested that the sensitivity and the resulting negative predictive value could be increased if the interpretation criteria were standardized and if fluorine-18-FDG PET were used to evaluate only thyroid nodules with a diameter larger than 1 cm (1,37).
Several recent studies, not included in the two meta-analyses, demonstrated the added value of incorporating fluorine-18-FDG PET/CT in the diagnostic work-up of cytologically indeterminate thyroid nodules (28,39,40).
A cost-effectiveness analysis demonstrated that implementation of a preoperative fluorine-18-FDG PET in patients with indeterminate thyroid nodules could prevent up to 47% of unnecessary surgical procedures, leading to a modest increase of health-related quality of life and cost reduction. When compared to in-vitro molecular tests, fluorine-18-FDG PET/CT showed similar effectiveness as that of gene-expression analyses (41).
In a visual comparison to technetium-99m-MIBI, fluorine-18-FDG PET demonstrated a higher diagnostic performance in discriminating between benign and malignant indeterminate thyroid nodules (28).
In addition to discriminating known thyroid lesions with fluorine-18-FDG PET, also the incidental fluorine-18-FDG uptake in the thyroid gland is a frequent diagnostic challenge. Such an uptake is found in 1–2% of patients, while an additional 2% of patients demonstrating diffuse thyroid uptake (2,42-44). Importantly, focal fluorine-18-FDG uptake is associated with an increased risk of malignancy, and therefore clinical evaluation and fine needle aspiration are recommended by the ATA for thyroid nodules greater than 1 cm with increased fluorine-18-FDG uptake (2).
Thyroid nodules smaller than 1 cm with increased fluorine-18-FDG uptake, on the other hand, can be monitored with ultrasound. A recent meta-analysis showed that 35% of fluorine-18-FDG positive nodules proved to be cancerous (42). In contrast, diffuse thyroid uptake most often represents benign disease corresponding to inflammatory uptake like Hashimoto’s disease or other diffuse thyroid illness.
Conclusions
Radioisotope imaging is an integral part of the work-up for discriminating benign from malignant cytologically indeterminate thyroid nodules (Table 1). While being associated with a certain radiation dose (Table 2), each imaging modality has a specific place in the clinic routine.

Full table

Full table
Thyroid scintigraphy with either iodine-123 or technetium-99m-pertechnetate remains the standard method to assess whether a nodule is autonomously functioning, and therefore whether nodules require further diagnostic evaluation.
The use of technetium-99m-MIBI scintigraphy is supported by robust clinical data as a second line procedure for the prediction of malignancy in cold nodules with indeterminate fine-needle cytologies, and the inclusion of this imaging method in future versions of clinical guidelines may require consideration.
Fluorine-18-FDG-PET/CT studies present controversial results in differentiating benign from malignant lesions in cytologically indeterminate thyroid nodules, and deserves further investigation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Giovanella L, Suriano S, Maffioli M, et al. 18FDG-positron emission tomography/computed tomography (PET/CT) scanning in thyroid nodules with nondiagnostic cytology. Clin Endocrinol (Oxf) 2011;74:644-8. [Crossref] [PubMed]
- Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract 2010;16:468-75. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017;27:1341-6. [Crossref] [PubMed]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Baker CH, Morris JC. The sodium-iodide symporter. Curr Drug Targets Immune Endocr Metabol Disord 2004;4:167-74. [Crossref] [PubMed]
- Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta 1993;1154:65-82. [Crossref] [PubMed]
- Kaminsky SM, Levy O, Salvador C, et al. The Na+/I- symporter of the thyroid gland. Soc Gen Physiol Ser 1993;48:251-62. [PubMed]
- Giovanella L, Ceriani L, Treglia G. Role of isotope scan, including positron emission tomography/computed tomography, in nodular goitre. Best Pract Res Clin Endocrinol Metab 2014;28:507-18. [Crossref] [PubMed]
- Giovanella L. Thyroid nodules: clinical management and differential diagnosis. Praxis (Bern 1994) 2009;98:83-90.
- Meller J, Becker W. Scintigraphic evaluation of functional thyroidal autonomy. Exp Clin Endocrinol Diabetes 1998;106 Suppl 4:S45-51. [Crossref] [PubMed]
- Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med 2004;351:1764-71. [Crossref] [PubMed]
- Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Paschke R, Hegedus L, Alexander E, et al. Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat Rev Endocrinol 2011;7:354-61. [Crossref] [PubMed]
- Verburg FA, Aktolun C, Chiti A, et al. Why the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 2016;43:1001-5. [Crossref] [PubMed]
- Meller J, Becker W. The continuing importance of thyroid scintigraphy in the era of high-resolution ultrasound. Eur J Nucl Med Mol Imaging 2002;29 Suppl 2:S425-38. [Crossref] [PubMed]
- Treglia G, Trimboli P, Verburg FA, et al. Prevalence of normal TSH value among patients with autonomously functioning thyroid nodule. Eur J Clin Invest 2015;45:739-44. [Crossref] [PubMed]
- Chami R, Moreno-Reyes R, Corvilain B. TSH measurement is not an appropriate screening test for autonomous functioning thyroid nodules: a retrospective study of 368 patients. Eur J Endocrinol 2014;170:593-9. [Crossref] [PubMed]
- Hurtado-Lopez LM, Monroy-Lozano BE, Martinez-Duncker C. TSH alone is not sufficient to exclude all patients with a functioning thyroid nodule from undergoing testing to exclude thyroid cancer. Eur J Nucl Med Mol Imaging 2008;35:1173-8. [Crossref] [PubMed]
- Ianni F, Perotti G, Prete A, et al. Thyroid scintigraphy: an old tool is still the gold standard for an effective diagnosis of autonomously functioning thyroid nodules. J Endocrinol Invest 2013;36:233-6. [PubMed]
- Schicha H, Hellmich M, Lehmacher W, et al. Nuklearmedizin 2009;48:79-83. [Should all patients with thyroid nodules > or = 1 cm undergo fine-needle aspiration biopsy?]. [Crossref] [PubMed]
- Reschini E, Ferrari C, Castellani M, et al. The trapping-only nodules of the thyroid gland: prevalence study. Thyroid 2006;16:757-62. [Crossref] [PubMed]
- Treglia G, Caldarella C, Saggiorato E, et al. Diagnostic performance of (99m)Tc-MIBI scan in predicting the malignancy of thyroid nodules: a meta-analysis. Endocrine 2013;44:70-8. [Crossref] [PubMed]
- Hurtado-Lopez LM, Arellano-Montano S, Torres-Acosta EM, et al. Combined use of fine-needle aspiration biopsy, MIBI scans and frozen section biopsy offers the best diagnostic accuracy in the assessment of the hypofunctioning solitary thyroid nodule. Eur J Nucl Med Mol Imaging 2004;31:1273-9. [Crossref] [PubMed]
- Wale A, Dizdarevic S. Combined (99m)Tc-methoxyisobutylisonitrile scintigraphy and fine-needle aspiration cytology offers an accurate and potentially cost-effective investigative strategy for the assessment of solitary or dominant thyroid nodules: reply to comments by Riazi et al. Eur J Nucl Med Mol Imaging 2014;41:577-8. [Crossref] [PubMed]
- Giovanella L, Campenni A, Treglia G, et al. Molecular imaging with (99m)Tc-MIBI and molecular testing for mutations in differentiating benign from malignant follicular neoplasm: a prospective comparison. Eur J Nucl Med Mol Imaging 2016;43:1018-26. [Crossref] [PubMed]
- Saggiorato E, Angusti T, Rosas R, et al. 99mTc-MIBI Imaging in the presurgical characterization of thyroid follicular neoplasms: relationship to multidrug resistance protein expression. J Nucl Med 2009;50:1785-93. [Crossref] [PubMed]
- Boi F, Lai ML, Deias C, et al. The usefulness of 99mTc-SestaMIBI scan in the diagnostic evaluation of thyroid nodules with oncocytic cytology. Eur J Endocrinol 2003;149:493-8. [Crossref] [PubMed]
- Piccardo A, Puntoni M, Treglia G, et al. Thyroid nodules with indeterminate cytology: prospective comparison between 18F-FDG-PET/CT, multiparametric neck ultrasonography, 99mTc-MIBI scintigraphy and histology. Eur J Endocrinol 2016;174:693-703. [Crossref] [PubMed]
- Campenni A, Giovanella L, Siracusa M, et al. (99m)Tc-Methoxy-Isobutyl-Isonitrile Scintigraphy Is a Useful Tool for Assessing the Risk of Malignancy in Thyroid Nodules with Indeterminate Fine-Needle Cytology. Thyroid 2016;26:1101-9. [Crossref] [PubMed]
- Theissen P, Schmidt M, Ivanova T, et al. MIBI scintigraphy in hypofunctioning thyroid nodules--can it predict the dignity of the lesion? Nuklearmedizin 2009;48:144-52. [Crossref] [PubMed]
- Campenni A, Siracusa M, Ruggeri RM, et al. Differentiating malignant from benign thyroid nodules with indeterminate cytology by (99m)Tc-MIBI scan: a new quantitative method for improving diagnostic accuracy. Sci Rep 2017;7:6147. [Crossref] [PubMed]
- Heinzel A, Muller D, Behrendt FF, et al. Thyroid nodules with indeterminate cytology: molecular imaging with (9)(9)mTc-methoxyisobutylisonitrile (MIBI) is more cost-effective than the Afirma gene expression classifier. Eur J Nucl Med Mol Imaging 2014;41:1497-500. [Crossref] [PubMed]
- Brown RS, Leung JY, Kison PV, et al. Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer. J Nucl Med 1999;40:556-65. [PubMed]
- Brown RS, Goodman TM, Zasadny KR, et al. Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl Med Biol 2002;29:443-53. [Crossref] [PubMed]
- Conti PS, Lilien DL, Hawley K, et al. PET and [18F]-FDG in oncology: a clinical update. Nucl Med Biol 1996;23:717-35. [Crossref] [PubMed]
- Haugen BR. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 2017;123:372-81. [Crossref] [PubMed]
- Vriens D, de Wilt JH, van der Wilt GJ, et al. The role of [18F]-2-fluoro-2-deoxy-d-glucose-positron emission tomography in thyroid nodules with indeterminate fine-needle aspiration biopsy: systematic review and meta-analysis of the literature. Cancer 2011;117:4582-94. [Crossref] [PubMed]
- Wang N, Zhai H, Lu Y. Is fluorine-18 fluorodeoxyglucose positron emission tomography useful for the thyroid nodules with indeterminate fine needle aspiration biopsy? A meta-analysis of the literature. J Otolaryngol Head Neck Surg 2013;42:38. [Crossref] [PubMed]
- Rosario PW, Rocha TG, Calsolari MR. Fluorine-18-fluorodeoxyglucose positron emission tomography in thyroid nodules with indeterminate cytology: a prospective study. Nucl Med Commun 2019;40:185-7. [Crossref] [PubMed]
- Buyukdereli G, Aktar Y, Kara E, et al. Role of 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Evaluation of Cytologically Indeterminate Thyroid Nodules. Iran J Radiol 2016;13:e21186. [Crossref] [PubMed]
- Vriens D, Adang EM, Netea-Maier RT, et al. Cost-effectiveness of FDG-PET/CT for cytologically indeterminate thyroid nodules: a decision analytic approach. J Clin Endocrinol Metab 2014;99:3263-74. [Crossref] [PubMed]
- Soelberg KK, Bonnema SJ, Brix TH, et al. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid 2012;22:918-25. [Crossref] [PubMed]
- Chen W, Parsons M, Torigian DA, et al. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nucl Med Commun 2009;30:240-4. [Crossref] [PubMed]
- Nishimori H, Tabah R, Hickeson M, et al. Incidental thyroid "PETomas": clinical significance and novel description of the self-resolving variant of focal FDG-PET thyroid uptake. Can J Surg 2011;54:83-8. [Crossref] [PubMed]


