
Role of multidetector computed tomography in the assessment of pancreatic injuries after blunt trauma: a multicenter experience
Introduction
Traumatic pancreatic injuries typically occur from acute penetrating or blunt abdominal trauma. They are considered rare and occur only between 0.2–12% of acute blunt abdominal trauma (1-5) with a mortality rate up to 30%, and a morbidity of 60% (1,4,6).
Because of its anatomic location and its relatively fixed position anterior to the spine, traumatic injuries to the pancreas are infrequent. Concomitant injuries are common (50–98%), with the liver most commonly affected (46.8% of cases) followed by stomach (42.3%), major vessels (41.3%), spleen (28.0%), kidney (23.4%), and duodenum (19.3%) (2). Since pancreatic injuries are an uncommon injury and are typically associated with other abdominal organ injuries, the diagnosis can be frequently missed (2).
The first step in order to avoid missing pancreatic injuries, is to consider the mechanism of trauma and then, evaluate the anatomical structures which are involved (7,8). Severe abdominal trauma antero-posteriorly directed, compressing the pancreatic gland against the spine, such as seat-belt injuries, acceleration-deceleration trauma, and handlebar compression trauma, represent the most common mechanism of injury (4). The most commonly injured segment of the pancreas is the body, which occurs in 65% of the cases (4).
The detection and grading of pancreatic injuries are important in order to facilitate a proper treatment plan (2,9).
Imaging of pancreatic injuries
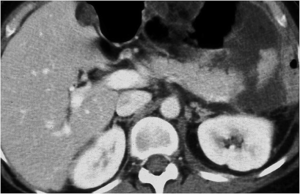
Multidetector computed tomography (MDCT) is the preferred imaging modality in patients with blunt trauma and, particularly, is the imaging modality of choice for the detection of acute pancreatic injury with a sensitivity of up to 80% (10-12). The MDCT sensitivity is variable as imaging findings are subtle within the first 12 hours of injury (2,13). This is secondary to the small amount of peripancreatic fat tissue that limit the MDCT detection of pancreatic injury, or with the time needed to develop imaging manifestations of post-traumatic inflammatory phenomena (Figure 1). As the presence of these kind of injuries affect the treatment, they deserve to be accurately identified, and so, in these cases, magnetic resonance (MR) imaging is indicated in combination with endoscopic retrograde cholangiopancreatography (ERCP) (9,14).
MDCT protocol
MDCT protocol in polytraumatized patients basically includes an unenhanced computed tomography (CT) of the head and an arterial and venous phase extended from the circle of Willis to the symphysis pubis (7).
Intravenous contrast material consists of 100–120 mL iodinated contrast agent at high iodine concentration 370–400 mg/mL, injected at 4–5 mL/s, followed by 40 mL of saline chaser at the same flow rate, to obtain optimal vessel depiction.
To time the beginning of the arterial phase, it is suggested the adoption of an automated bolus tracking, with region of interest placed in the aortic arch at an attenuation threshold of 100 HU. The venous phase was performed at 60–70 s delay from the end of the injection. A delayed, excretory phase (180 s delay from the end of the venous phase or later) can be added in select cases, for further evaluation of suspected slight bleeding or if there was suspected injury to the collecting system or bladder.
All initial MDCT scans for trauma patients are obtained without oral contrast material. The use of oral contrast material is reserved for follow-up CT studies or “second look” evaluations. In the follow-up CT of patients with suspected or diagnosed pancreatic trauma, it can be useful to obtain an adequate distension of the duodenal lumen to facilitate the depiction of possible coexistent injuries in this area.
The examination should be obtained with thin slice thickness (1.2 mm), thinner reconstructed (0.625 mm) to allow for optimal three-dimensional multiplanar reconstructions (MPR). The use of MPR, maximum intensity projection (MIP) and minimum intensity projection (MinIP) is critical in assessing for biliary duct anomalies.
MDCT findings
Signs of pancreatic injuries may be indirectly suspected or directly depicted at imaging.
Indirect signs may be considered the trajectory of injury through the region of the pancreas and the presence of peripancreatic fat stranding (1).
Direct signs at CT scans may vary from contusion, edema or hematoma of the pancreatic parenchyma, lacerations or fractures of the pancreas (14).
Pancreatic contusion is seen as focal or diffuse area of low attenuation within the normally enhancing pancreas. It can be defined as minor when involves one focal region of the organ (uncinate process/head/isthmus/body/tail) or major when more than one region is involved (5).
Pancreatic edema depends on the edematous infiltration of the parenchyma due to inflammatory phenomena following trauma (Figure 1) (13).
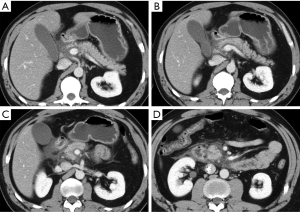
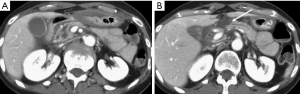
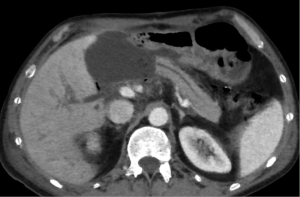
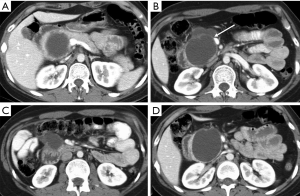
Hematoma represents a blood collection that may be limited to the parenchyma, or may extend in the peripancreatic tissue, and in severe cases can be associated with active bleeding. Large hematomas may also cause mass effect obstructing the duodenal lumen (Figure 2) or the pancreatic duct (5,15).
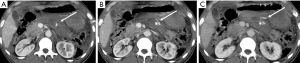
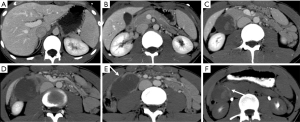
Pancreatic laceration is identified as a low-density line or band oriented perpendicular or oblique to the long axis of the gland (5) (Figures 3,4). Depending on the extension, lacerations may be superficial (less than 50% depth or thickness of the gland) or deep (beyond 50% of the depth of the gland reaching up to the plane of the main pancreatic duct and thus likely to be involving the duct). The pancreatic duct is inconsistently visualized directly on MDCT and a laceration involving more than 50% of the depth of the pancreas is taken as indirect evidence of ductal injury on CT (5) (Figure 4).
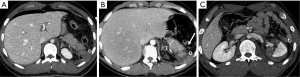
Extensive lacerations may lead to complete transection/fracture of the gland (5) (Figures 4,5).
Once pancreatic injury is detected, it is important to evaluate its severity, as it correlates with the need for surgery and with the rate of complications (16), and the presence of coexistent injuries.
To describe the injury grade in the report, it is suggested the adoption of the AAST classification, thus ensuring a uniform communication of the relevant findings (17), related with injuries of the pancreatic duct or of the pancreatic head (for the involvement of the ampulla), as they change the patient prognosis and management (Figures 6,7) (9).
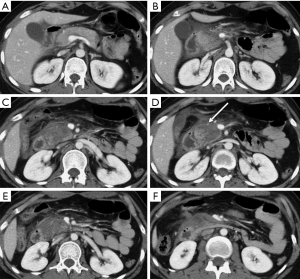
AAST classification considers as grade I minor contusion or superficial lacerations without duct injury, grade II major contusion or laceration without duct injuries or tissue loss, grade III distal transection or parenchymal injury with duct injury, grade IV proximal transection or parenchymal injury involving ampulla, grade V massive disruption of the pancreatic head (17).
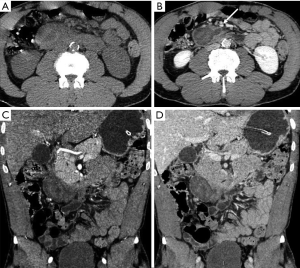
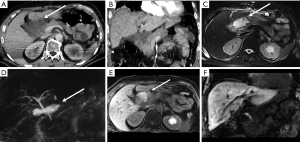
However, there are further injuries not considered in the AAST classification, deserving an accurate evaluation at admission MDCT after trauma due to their importance in patient’s survival: vascular injuries. To properly identify and characterize vascular injuries it is recommended the acquisition of at least two phases (arterial and portal venous) after IV injection (Figure 8) (8,18,19). This allows the radiologist to depict the origin (arterial or venous) and to estimate the entity of the bleeding, assuming a crucial role in the patient’s management, as slight venous bleeding may be conservatively treated in stable patients, whereas jet or pooling of active bleeding need to be managed by endovascular approach, if possible, in arterial bleeding, or by surgical approach (7,18) (Figure 9).
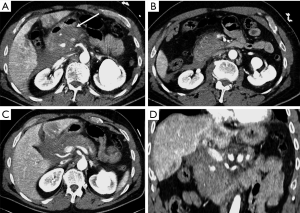
In trauma patients with persisted hypoperfusion, may be encountered alterations of pancreatic enhancement known as “shock pancreas”. The pancreas may demonstrate hypoenhancement in early phase or hyperenhancement (20-24). The decreased enhancement depends on the reduced blood supply and may cause difficulties in discerning from pancreatic injury, although these injuries are usually more focal (20). The pathophysiological causes of increased density are still not well known, and may depend on the decompensation in the blood flow regulation. The persistent pancreatic hyperdensity is more frequently observed in critical shocked patients and is related with poor prognosis (20,21,25) (Figure 10).
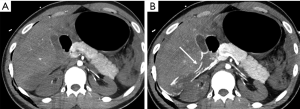
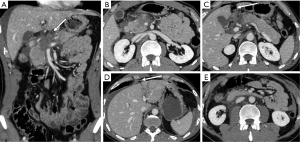
A crucial point to be considered in the evaluation of parenchymal injury is the involvement of the pancreatic duct, as ductal injuries deserve to be operatively approached (Figure 11), and lead to a major percentage of complications (Figure 12). Indeed, the risk of development of abscess or fistula in patients with disruption of the pancreatic duct is 25% and 50%, respectively, in comparison with 10% without duct injuries (13). However, the accuracy of MDCT in the detection of ductal injury has been reported to be as low as 43%, for this reason the pancreatic duct is indirectly considered as injured on MDCT if the parenchymal laceration involves more than 50% of the depth of the pancreas. In such cases a magnetic resonance cholangiopancreatography (MRCP) or ERCP is recommended (5,13). Sometimes the ductal injury may also be seen at CT, this is made easier with the adoption of MPR and MinIP (Figures 11,12).
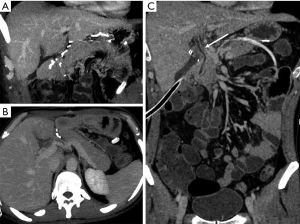
The primary pitfall in the diagnosis of pancreatic trauma is related with the presence of parenchymal cleft mimicking lacerations (Figure 13) (4).
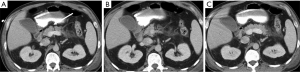
When there is a pancreatic involvement in trauma, it is particularly important to carefully evaluate the duodenum (Figure 14), as it is frequently injured together with the pancreas (Figure 15). Also imaging findings of duodenal injury may be subtle and a slight thickness of the wall or the presence of small air bubbles in the adjacent extraluminal spaces may represent signs of injury (4) (Figure 4).
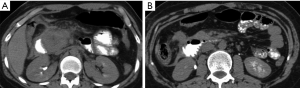
Complications
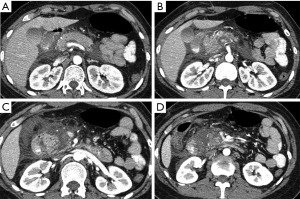
Complications of pancreatic injuries develop in up to one-third of the patients and include pancreatitis, pseudocysts, fistulas, intra-abdominal abscesses, or bowel anastomosis breakdown, and may lead to sepsis and multiorgan failure (16). Post-traumatic leakage of pancreatic enzymes may lead to pseudocyst formation (Figures 12,16,17) with further complication as abscesses, or may predispose to abnormal fistulization between the pancreas and adjacent organs. Furthermore, leakage of enzymes may lead to vascular wall erosion forming pseudoaneurysm that may complicate with delayed hemorrhage (16).
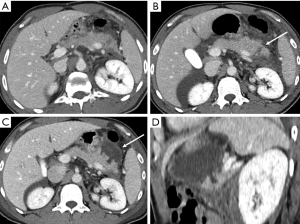
Pseudoaneurysm development after trauma may also depend on variation in the blood flow following stenosis or occlusion of the celiac or hepatic arteries (26).
Post-traumatic strictures of the pancreatic duct, which can predispose to recurrent pancreatitis, have also been reported (4).
Follow-up
Due to low sensitivity of MDCT in early phases of trauma, it is important to select an appropriate imaging modality for follow-up. In our Institutions, the imaging modality of choice for follow-up is MR. MR provides a high contrast resolution, the multiplanar and good spatial resolution without using IV (27). First MR follow-up is usually performed between 48 and 60 hours from trauma. Imaging protocol basically consists in: T2W sequences acquired in axial, coronal and, if needed, sagittal planes, to evaluate the presence of fluid collections, T1W fat sat and T1W in and out sequences in axial planes to explore the pancreatic parenchyma and the presence of blood collections, and the MRCP sequences to evaluate the ductal injury and the relationship with peripancreatic fluid collections (Figure 18). The acquisitions of post-contrast T1W fat sat sequences helps in the evaluation of vascular complications and in the estimation of pancreatic lacerations and necrosis (5,28). Furthermore, the use of hepatobiliary contrast agent [gadobenate dimeglumine (Gd-BOPTA), MultiHance, Bracco; or gadoxetic acid (Gd-EOB-DTPA), Primovist in Europe; Eovist in the USA; Bayer Healthcare] may help in the evaluation of concomitant adjacent biliary duct injuries (Figure 9). The timing of further follow-up examinations depends on the injuries detected and on the therapeutic choices. In patients with injuries in multiple anatomical districts, i.e. deserving also the evaluation of major thoracic injuries, MDCT remains the modality of choice also for the follow-up (Figure 19).
Conclusions
Pancreatic injuries which occur due to high energy blunt abdominal trauma are rare and commonly are associated with abdominal organ injuries. Contrast-enhanced MDCT represents the imaging modality of choice immediately after trauma, allowing a complete and fast evaluation, even though sometimes it has a low sensitivity. So, in these cases, in the pre-operative assessment and in the patient follow-up, is indicated the additional use of MR and MRCP, due to its high contrast resolution properties particularly useful for the study of the pancreatobiliary tract.
Acknowledgements
The Authors are grateful to Giovanna Casola and Claude Sirlin of the University of California San Diego, USA, for their contribution.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gordon RW, Anderson SW, Ozonoff A, et al. Blunt pancreatic trauma: evaluation with MDCT technology. Emerg Radiol 2013;20:259-66. [Crossref] [PubMed]
- Scaglione M, Romano L, Bocchini G, et al. Multidetector computed tomography of pancreatic, small bowel, and mesenteric traumas. Semin Roentgenol 2012;47:362-70. [Crossref] [PubMed]
- Dreizin D, Bordegaray M, Tirada N. Evaluating blunt pancreatic trauma at whole body CT: current practices and future directions. Emerg Radiol 2013;20:517-27. [Crossref] [PubMed]
- Melamud K, LeBedis CA, Soto JA. Imaging of Pancreatic and Duodenal Trauma. Radiol Clin North Am 2015;53:757-71. [Crossref] [PubMed]
- Panda A, Kumar A, Gamanagatti S. Evaluation of diagnostic utility of multidetector computed tomography and magnetic resonance imaging in blunt pancreatic trauma: a prospective study. Acta Radiol 2015;56:387-96. [Crossref] [PubMed]
- Lin BC, Wong YC, Chen RJ, et al. Major pancreatic duct continuity is the crucial determinant in the management of blunt pancreatic injury: a pancreatographic classification. Surg Endosc 2017;31:4201-10. [Crossref] [PubMed]
- Schueller G, Scaglione M, Linsenmaier U. The key role of the radiologist in the management of polytrauma patients: indications for MDCT imaging in emergency radiology. Radiol Med 2015;120:641-54. [Crossref] [PubMed]
- Scaglione M, Iaselli F, Sica G, et al. Errors in imaging of traumatic injuries. Abdominal Imaging 2015;40:2091-8. [Crossref] [PubMed]
- Søreide K, Weiser TG, Parks RW. Clinical update on management of pancreatic trauma. HPB (Oxford) 2018;20:1099-108. [Crossref] [PubMed]
- Vasquez M, Cardarelli C, Glaser J, et al. The ABC's of Pancreatic Trauma: Airway, Breathing, and Computerized Tomography Scan? Mil Med 2017;182:66-71. [Crossref] [PubMed]
- Moschetta M, Telegrafo M, Malagnino V, et al. Pancreatic trauma: The role of computed tomography for guiding therapeutic approach. World J Radiol 2015;7:415-20. [Crossref] [PubMed]
- Giovine S, Romano L, Rossi G, et al. (Computed tomography in the diagnosis of posttraumatic pancreatic lesions). Radiol Med 1997;94:341-5. [PubMed]
- Debi U, Kaur R, Prasad KK, et al. Pancreatic trauma: A concise review. World J Gastroenterol 2013;19:9003-11. [Crossref] [PubMed]
- Linsenmaier U, Wirth S, Reiser M, et al. Diagnosis and classification of pancreatic and duodenal injuries in emergency radiology. Radiographics 2008;28:1591-602. [Crossref] [PubMed]
- Fu CY, Wu SC, Chen RJ, et al. Blunt pancreatic head hematoma as an infrequent cause of delayed obstructive jaundice. J Emerg Med 2012;42:e27-9. [Crossref] [PubMed]
- Byrge N, Heilburn M, Winkler N, et al. An aast-mitc analysis of pancreatic trauma: staple or sew? resect or drain? J Trauma Acute Care Surg 2018;85:435-43. [PubMed]
- Moore EE, Cogbill TH, Malangoni MA, et al. Scaling system for organ specific injuries. Curr Op Crit Care 1996;2:450-62. [Crossref]
- Iacobellis F, Ierardi AM, Mazzei MA, et al. Dual-phase CT for the assessment of acute vascular injuries in high-energy blunt trauma: the imaging findings and management implications. Br J Radiol 2016;89:20150952. [Crossref] [PubMed]
- Sica G, Guida F, Bocchini G, et al. Errors in imaging assessment of polytrauma patients. Semin Ultrasound CT MR 2012;33:337-46. [Crossref] [PubMed]
- Lubner M, Demertzis J, Lee JY, et al. CT evaluation of shock viscera: a pictorial review. Emerg Radiol 2008;15:1-11. [Crossref] [PubMed]
- Higashi H, Tamada T, Kanki A, et al. Hypovolemic shock complex: does the pancreatic perfusion increase or decrease at contrast-enhanced dynamic CT? Clin Imaging. 2014;38:31-4. [Crossref] [PubMed]
- Lamy M, Faymonville ME, Deby-Dupont G. Shock Pancreas: A New Entity? In: Vincent JL. editor. Update in Intensive Care and Emergency Medicine, vol 3. Springer, Berlin, Heidelberg, 1987:148-54.
- Ames JT, Federle MP. CT hypotension complex (shock bowel) is not always due to traumatic hypovolemic shock. AJR Am J Roentgenol 2009;192:W230-5. [Crossref] [PubMed]
- Wang J, Liang T, Louis L, et al. Hypovolemic shock complex in the trauma setting: a pictorial review. Can Assoc Radiol J 2013;64:156-63. [Crossref] [PubMed]
- Tarrant AM, Ryan MF, Hamilton PA, et al. A pictorial review of hypovolaemic shock in adults. Br J Radiol 2008;81:252-7. [Crossref] [PubMed]
- Prosper A, Saremi F, Angeles L. Delayed Development of Multiple Pancreaticoduodenal Arcade Pseudoaneurysms after Abdominal Trauma. Ann Vasc Surg 2016;36:297.e11-5. [Crossref] [PubMed]
- Ragozzino A, Manfredi R. The use of MRCP in the detection of pancreatic injuries after blunt trauma. Emerg Radiol 2003;10:14-8. [PubMed]
- Girard E, Abba J, Cristiano N, et al. Management of splenic and pancreatic trauma. J Visc Surg 2016;153:45-60. [Crossref] [PubMed]


