
Bleeding after prostatectomy: endovascular management
Introduction
American Cancer Society reported that about one man in nine will be diagnosed with prostate cancer during his lifetime (1,2).
Current treatment with curative aim is radical prostatectomy (RP) a surgical intervention that consists in removing entire prostate gland, seminal vesicles and vas deferens with nodes close to the gland, otherwise extended pelvic lymph node dissection (ePLD) is reserved for patients with higher risk of nodal involvement based on controversial literature criteria like explained in Briganti et al. (3).
Prostate surgery can be performed with different technical approaches: open RP (ORP), “conventional” laparoscopic (LRP) or robotic-assisted laparoscopic (RA-LRP) that is currently preferred for its minimally surgical invasiveness in case of localised prostate cancer (4).
Despite the insufficiency of uniformity in documenting and reporting complications caused by different surgical approaches, Rabbani et al. reported most of RP’s complications and grouped them into two subgroups: medical and surgical one (5) (Table 1).
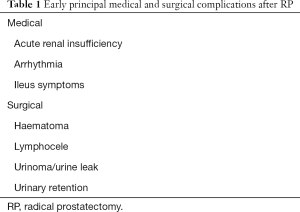
Full table
One of the feared surgical major complication is gross bleeding and pelvic haematoma, more frequent in case of ORP and LRP, but also in RA-LRP may occurs (6-9).
Typically blood loss starts from an injured vessel in surgical area and generally is self-limiting, however intervention is necessary when occurs conspicuous hemorrhage.
Radiologic examinations to detect postoperative urologic complications include ultrasound, as first line method, providing quickly and global informations of urinary system and detecting free fluid in pelvis or in abdominal space collecting (10).
Computed tomography (CT) imaging should be obtained for comprehensive view of all extraprostatic tissues. Due to its intrinsical high spatial resolution, contrast medium CT [multidetector-row CT (MDCT)] is the gold standard for identification of blood collections, extravasated urine and active bleeding (11-13).
In evaluation of postoperative urologic patients standard MDCT protocol must contained unenhanced starting acquisition to detect hyperintensity images and free abdominal air; recent haematoma usually shows 45–75 HU attenuation in unenhanced scans due to high protein content with hyperdense appearance comparatively to muscles.
After contrast medium injection enhanced imaging must evaluated arterial- and parenchymal-phase for development of extravasal distribution and excretory phase in order to assess iodinated urine leaks and urinomas (10).
Compared to surgical explorative approach, current standard of care, endovascular treatment of significative postsurgical prostate hemorrhage has been demonstrated to be high accurate and minimally invasive reducing morbidity with shorter hospital stay and more rapid recovery (14).
The present series includes severe haemorrhagic patients after RP that were successfully treated by transarterial approach. Technical and clinical success, safety and long-term outcomes were analysed.
Methods
Our Internal Review Board approved the retrospective revision of the cases.
Written informed consent was obtained, when possible. The study was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guideline on Good Clinical Practice, and relevant local laws and regulations.
We evaluated data of 10 transarterial embolizations in patients undergoing RP characterized by significative postprocedural bleeding diagnosed between April 2015 and January 2018 (Table 2).
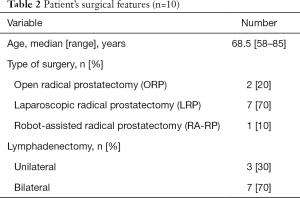
Full table
Bleeding was clinically evaluated with signs and symptoms of vascular injury, such as decreasing in systolic blood pressure to 100 mmHg or less and/or tachycardia more than 100 beats per minute, and with laboratory findings such as haemoglobin reduction of at least 2 g/dL.
The mean time between endovascular treatment was 3.6 days (1–7 days) and all patients were evaluated by an emergency angio-CT that confirmed the presence of haemorrhage (6 pseudoaneurysms and 4 blushing).
On digital subtraction angiography (DSA) bleeding vessel was detected: a side branch of pudendal artery was responsible in 9 patients and in one case a prostatic side branch of inferior gluteal artery was the haemorrhagic vessel.
Procedure
All procedures were performed in AngioSuite (GE-Innova 2100-IQ, GE Healthcare, USA; Allura Xper FD20 with flat panel detector, Philips, Best, The Netherlands; Siemens Artis Zee, Erlangen, Germany) under local anesthesia and with anesthesiological assistance.
From common femoral artery approach, we catheterized internal iliac artery (IIA) bilaterally and we performed angiography.
A superselective catheterization of the branches of both pudendal arteries with a 2.7-F microcatheter (Progreat, Terumo, Tokyo, Japan) was obtained.
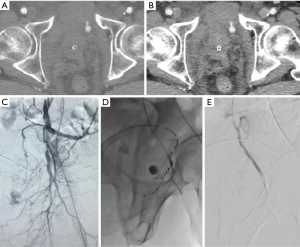
Six pseudoaneurysms (Figure 1A,B,C,D,E) and 4 active bleedings (Figure 2A,B,C) were revealed.

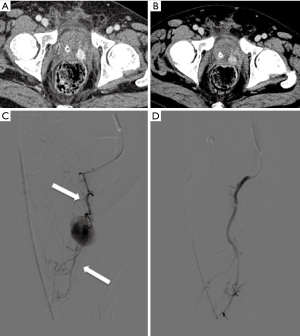
A superselective embolization was obtained using microcoils, onyx and spongostan until the stop of arterial flow (Figure 3A,B,C,D).
Femoral sheath was left for 24–48 hours until haemodynamic and laboratory data were stable.
Outcomes
Technical success, clinical success and complications were evaluated.
Technical success was defined by exclusion of bleeding, and restoration of peripheral flow.
Early clinical success was defined as cessation of symptoms and stabilization of laboratory data within 24 h and again within 1 week after endovascular procedure (i.e., absence of recurrent decrease of hemoglobin by <2 g/dL, circulatory stabilization).
Late success was defined as absence of reperfusion of bleeding during follow-up, and the proportion of cases that did not require endovascular repeat treatment or subsequent surgical intervention.
All complications were recorded and classified according to Society of Interventional Radiology classification (15).
Follow-up
All patients were closely monitored (symptoms and laboratory data) every 6 hours in the first 48 hours and 1 week after the endovascular procedure.
A new CT scan was performed to rule out new and/or residual bleeding, pseudoaneurysms or fistulas in cases of marginal haemodynamic stabilisation (n=1); in the latter case new bleeding was not diagnosed.
The endovascular procedure should be repeated, if the indication persists.
The interval between completion of the intervention and first imaging follow-up was in the range of 3 days–2 months. A minimum 8 months follow-up was available for all of our patients who underwent embolization.
Results
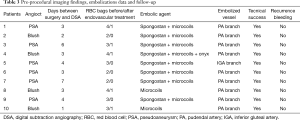
Technical success rate was 100% as shown by complete exclusion of bleeding on angiography performed at the end of the procedure (Table 3).
Full table
After procedure, in all patients, return to hemodynamic parameters was obtained with increased blood pressure and normalization of peripheral pulses.
No patient required conversion to open surgery and none required a second treatment, whether surgical or endovascular.
Clinical success, early and late, attributed to endovascular therapy alone was documented in every patient (100%). During follow-up (8–20 months) no recurrence of bleeding or sequelae related to non-target embolization were registered; one patient underwent to a new CT scan for marginal haemodynamic stabilization: new bleeding was not diagnosed.
Mean of red blood cell bags administered before embolization was 3 compared to 0.5 after endovascular treatment.
No major complications requiring intensive care were encountered during or after the procedure.
No minor complications were registered; mild post embolization syndrome with nausea, fever and slight pelvic pain, was registered in 2 patients.
Discussion
Severe post-operative haemorrhage following RP has been reported in 0.5% to 1.6% of cases and according to a range of definitions this complication can be treated by conservative, surgical or endovascular technique (14).
Using conservative approach the most important problems are hemodynamic shock and pelvic haematoma which is likely to result in mild/long-term urinary incontinence (16).
Surgical treatment requires further general anesthesia and refined search of small bloody vessels. Literature has shown that transarterial embolization makes shorter hospital stay compared to surgical approach (17).
Our retrospective study shows that endovascular approach is feasible and fast in case of arterial bleeding.
We achieved technical success of 100%, in absence of non-target embolization (pelvic ischemia complications), using like embolic agents microcoils (detachable coil) and spongostan; an early or late clinical success was documented in every patient at the first time; no recurrence bleeding was detected, worsening of clinical data was never observed and all patients improved during follow-up.
First studies have shown that risk of pelvic re-bleeding is higher if the embolization is unilateral compared to bilateral embolization (18,19); we performed a superselective catheterization of the branches of both pudendal arteries and we evaluated, based on pretreatment imaging and DSA, if whether to perform a mono or bilateral embolization. Embolization of branches of the pudendal artery was performed in nine patients and in one case a prostatic side branch of the inferior gluteal artery was the hemorrhagic vessel.
No case required a second embolization or open surgery conversion to stop bleeding; only in one case a new angio CT was performed for late return of physiological haemodynamic parameters, nevertheless no post-embolization bleeding was detected.
Mean of red blood cell bags administered before embolization was 3 compared to 0.5 after endovascular treatment.
Summarizing, our study shows that arterial embolization was useful and minimally invasive treatment: no major complications requiring intensive care were encountered during or after procedure and no minor complications were registered; only mild post embolization syndrome was registered in 2 patients with nausea, fever and slight pelvic pain during follow up (median =15 months; range, 8–20 months).
Moreover transarterial embolization became safer even more after development of new devices such as detachable microcoils with reduction of non-target embolizations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our Internal Review Board approved the retrospective revision of the cases (No. 2018C4455). The study was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guideline on Good Clinical Practice, and relevant local laws and regulations. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Briganti A, Larcher A, Abdollah F, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol 2012;61:480-7. [Crossref] [PubMed]
- Ontario Health Quality. Robotic Surgical System for Radical Prostatectomy: A Health Technology Assessment. Ont Health Technol Assess Ser 2017;17:1-172. [PubMed]
- Rabbani F, Yunis LH, Pinochet R, et al. Comprehensive standardized report of complications of retropubic and laparoscopic radical prostatectomy. Eur Urol 2010;57:371-86. [Crossref] [PubMed]
- Hu JC, Nelson RA, Wilson TG, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol 2006;175:541-6; discussion 546. [Crossref] [PubMed]
- Donat SM. Standards for surgical complication reporting in urologic oncology: time for a change. Urology 2007;69:221-5. [Crossref] [PubMed]
- Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int 2009;103:448-53. [Crossref] [PubMed]
- Fischer B, Engel N, Fehr JL, et al. Complications of robotic assisted radical prostatectomy. World J Urol 2008;26:595-602. [Crossref] [PubMed]
- Tonolini M, Villa F, Bianco R. Multidetector CT imaging of post-robot-assisted laparoscopic radical prostatectomy complications. Insights Imaging 2013;4:711-21. [Crossref] [PubMed]
- Yablon CM, Banner MP, Ramchandani P, et al. Complications of prostate cancer treatment: spectrum of imaging findings. Radiographics 2004;24 Suppl 1:S181-94. [Crossref] [PubMed]
- Gayer G, Zissin R, Apter S, et al. Urinomas caused by ureteral injuries: CT appearance. Abdom Imaging 2002;27:88-92. [Crossref] [PubMed]
- Kekelidze M, Dwarkasing RS, Dijkshoorn ML, et al. Kidney and urinary tract imaging: triple-bolus multidetector CT urography as a one-stop shop--protocol design, opacification, and image quality analysis. Radiology 2010;255:508-16. [Crossref] [PubMed]
- Hiroshige T, Matsuo M, Ueda K, et al. Transarterial embolization for pelvic hematoma following laparoscopic radical prostatectomy: A case report and review of the literature. Oncol Lett 2015;10:1889-92. [Crossref] [PubMed]
- Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14:S199-202. [Crossref] [PubMed]
- Kaufman JD, Lepor H. Reoperation versus observation in men with major bleeding after radical retropubic prostatectomy. Urology 2005;66:561-5. [Crossref] [PubMed]
- Beaujeux R, Saussine C, al-Fakir A, et al. Superselective endo-vascular treatment of renal vascular lesions. J Urol 1995;153:14-7. [Crossref] [PubMed]
- Hald T, Mygind T. Control of life-threatening vesical hemorrhage by unilateral hypogastric artery muscle embolization. J Urol 1974;112:60-3. [Crossref] [PubMed]
- Hietala SO. Urinary bladder necrosis following selective embolization of the internal iliac artery. Acta Radiol Diagn (Stockh) 1978;19:316-20. [Crossref] [PubMed]


