Biliary injuries after pancreatic surgery: interventional radiology management
Introduction
Pancreatic surgery is the primary therapy in various hepatobiliary and pancreatic diseases. Despite the improvements achieved in surgical techniques, biliary injuries remain a critical problem and a major cause of morbidity. The difficult location of the pancreas gland due to the presence of vital structures that surround it and the creation of a new anastomosis between the biliary and the gastrointestinal system, add complexity to pancreatic surgery (1-4).
In the past two decades, post-operative mortality after pancreatic surgery has decreased (2–5%), however, the incidence of post-operative complications remains high (30–60%) (5-8). Postoperative morbidities such as biliary anastomotic stricture, formation of gallstones, abscesses and bilomas, whose incidence range from 3% to 14%, may lead to later interventions (3,8,9). Bile leakage, biliary duct obstruction or stricture and infection represent the most common complications after surgery (8,9). Proper diagnosis and treatment of surgical biliary injuries (SBI) are paramount in preventing the life-threatening complications of cholangitis, intra-abdominal infection, biliary cirrhosis, end-stage liver disease and possibly resulting in death (10).
In many cases a definite diagnosis of a suspected postoperative complication needs a radiological confirmation. CT, MRI and ultrasound are valid methods in the diagnosis of SBI (3,8). Since the 1970’s, when the first image-guided percutaneous abscess drainage (PAD) was performed, there was a constant development of technical advices and techniques, leading to more treatment opportunities and better outcomes in interventional radiology (IR). To date radiological interventions play a pivotal rule and are considered the first-line management of postoperative complications optimizing the patient’s condition often resulting in a definitive treatment (5-7,11).
Biliary leakage
The definition of postoperative biliary leakage still remains arbitrary. Most definitions are based on cut-off values for the volume of drain fluid, ranged from 20 to 50 mL, and/or the bilirubin concentration within the drain fluid, from 5 to 20 mg/d, including certain time intervals that varied between 24 hours and 14 days after operation (1).
Biliary leakage may originate from injury of the bile ducts, or from anastomotic leakage after bilio-enteric anastomosis. Literature shows an incidence from 2% to 10% after pancreatic surgery without iatrogenic biliary injury; if biliary injury occurred incidence may rise up to 24% (Table 1) (1,3,4,11-15). A multivariate analysis has shown that there are no independent risk factors to predict bile leakage (4).

Full table
Placement of transpupillary plastic stents, biliary sphincterotomy alone, or a combination of both, is the first-line treatment for biliary leaks (8). However, after radical surgery, the endoscopic approach is limited and therefore it is necessary to proceed with percutaneous procedures to avoid a new surgical approach.
All patients with a high grade of bile leakage can be considered candidates for IR procedures, including percutaneous drainage of intra-abdominal fluid collections, percutaneous transhepatic cholangiography (PTC) and percutaneous transhepatic biliary drainage (PTBD).
PTC and PTBD
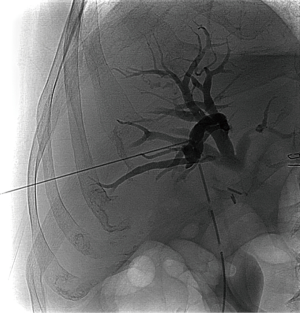
PTC is a diagnostic interventional radiology procedure that requires the sterile placement of a fine needle into peripheral biliary tree under fluoroscopic guidance; the injection of contrast material needs to delineate biliary anatomy and potential pathologic processes (Figure 1) (16).
The patients are prepared with high-spectrum antibiotic to avoid the risk of cholangitis and sepsis. Intravenous analgesics are usually administered just before and during the procedure, and the patient is usually under mild sedation (17). The puncture of the bile duct can be performed by ultrasound or fluoroscopic guidance. Site of puncture should be as peripheral as possible because central puncture have higher risk for major vascular injury. The cholangiography is decision-making to plan treatment (11,17).
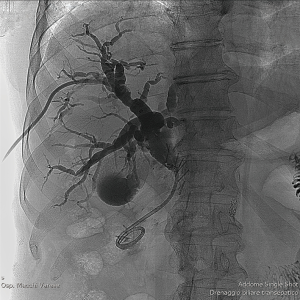
PTBD is the only endoluminal treatment option for biliary leakage when duodenum and common bile duct cannot be cannulated. It gives the opportunity to create a low-pressure system along the biliary tract, redirect the bile flow from the defect into the bile ducts allowing the leak to heal, to avoid further surgery or as a bridging therapy while stabilizing the patient’s condition prior to surgery (11).
Using dedicated guiding and catheter systems, under fluoroscopic guidance, a tube or stent is placed for external and/or internal drainage to cover the biliary tract (Figure 2) (16).
When the biliary leak persists, some safe and effective interventional procedures may be considered in relation to the characteristics of the bile injury. In particular, the biliary duct isolated can be occluded by the use of embolizing agents, such as fibrin, acetic acid, ethanol and glues (18). On the other hand, stent graft, coils or plug can be used to embolize leakage or fistula communicating with the biliary tree (Figure 3) (17,19-21).
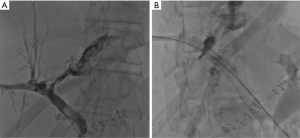
Allergic reactions to contrast agent and large volumes of ascites are relative contraindications; instead, uncontrollable coagulation status and lack of a safe access route are the only severe contraindications (11,16,21).
In patients with persistent coagulopathy, these procedures may still be indicated if they are associated with a lower expected morbidity rate than alternative methods of diagnosis or treatment (16).
Technical success and complications
Successful PTC is defined as sufficient needle localization and contrast material opacification to allow image-based diagnosis or planning of treatment. Successful PTBD is defined as the placement of a tube or stent with use of imaging guidance to provide continuous drainage of bile (16).
In patients with non-dilated ducts, technical success of PTC has been reported between 65% and 90% (11). Success rates of PTBD for biliary leakage are reported to be 40–100% without secondary surgery (Table 2) (11,22-27).

Full table
Sohn et al. [2003] preformed PTC and/or PTBD procedures on 39 patients with postoperative biliary leaks. The procedures were successful in 32 out of the 39 patients treated, with a success rate of 82% (13). Popat et al. [2017] preformed PTBD procedures on 157 patients with postoperative biliary leaks between the years 2002–2014. The procedure was successful in 62 out of the 157 patients treated, with a success rate of 40%.
The intrahepatic bile ducts were dilated in 17 patients (11%) and non-dilated in 140 patients (89%) (22). Mastier et al. [2018] preformed PTBD procedures on 101 patients with postoperative biliary leaks. The procedure was successful in 75 out of the 101 patients treated, with a success rate of 74% (24).
The average time range from PTBD placement to resolution of the leak is described as 9–150 days. In patients with biliary leakage, the biliary ducts are often non-dilated compared to biliary obstruction and the complication rate is reported to be higher (11,28).
The rate of major complications after PTC and PTBD is 2% to 4% and up to 25%, but there is a lack of differentiation between major and minor complications (11).
Major complications may be haemorrhage secondary to arterial injury as well as subcapsular liver hematoma, residual biliary stenosis, ductal perforation with risk for biliary peritonitis, sepsis, cholangitis, pancreatitis, metabolic acidosis from continuous bile loss have been also reported. A minor complication could be fever with chills, pain and peri-catheter leak (11,16,17).
Patients with coagulopathies, cholangitis, stones, malignant obstruction, or proximal obstruction will have higher complication rates (8,11,16,17).
Bilioenteric stricture
Anastomotic stricture is defined as a radiologically demonstrated narrowing of the biliary-enteric anastomosis associated with symptoms (jaundice, cholangitis or deranged liver function tests) requiring percutaneous, endoscopic or surgical treatment (3,29). Post-operative biliary stricture usually presented on an average period of 2.3 years (range, 0–15.3 years) after surgery, without any relationship with the nature of the primary disease (29,30).
Bile duct stenosis occurs within 30 days from surgery, predominantly with symptoms such as fever and sepsis, otherwise with asymptomatic jaundice (8). In cases of complete stenosis of the common bile duct, an increase of intraductal pressure is observed with bile loss from the site of the anastomosis and consequent peritonitis.
The incidence of biliary stricture formation in patients with benign and malignant disease ranges from 2.0% to 3.6% (Table 3) (3,4,29). The diameter of the common bile duct ≤5 mm represents a risk factor for bilio-enteric stenosis. Another factor associated with stenosis is the use of suture thread (6/0) for anastomosis (4).

Full table
The non-invasive diagnosis of biliary stricture can be performed by ultrasound, computed tomography and magnetic resonance imaging. Without contraindications, endoscopic retrograde cholangiography is the gold standard invasive diagnostic procedure. Cholangiography can establish ductal stenosis, identify its level and recognize the nature of the lesion (8,16).
PTC allows the subsequent placement of biliary drainage, dilation of stenosis, extraction of calculi if present or placement of a stent through severe stenosis.
The interventional treatment depends on the degree of injury, the presence of complications and the operational risk of the patient (8,21). Once the stenosis has been overcome, a decision whether to attempt balloon dilation should be made. The period between the creation of the anastomosis and the leak may be considered; when less than 1 month, leakage may be related to post-surgical oedema or a kink. Balloon dilation may be effective.
Balloon dilatation of focal segment strictures determines the patency of short- and long-term stenosis of 50–90% and 56–74% respectively. Instead, strictures of long or multifocal segments do not respond to dilatation (21).
The diameter of the balloon must be oversized by 25–30%. The balloon size usually varies from 10 to 14 mm in the main ducts and 4 to 8 mm in the distal bile ducts. The dilation must be repeated several times in the site of stenosis with different duration (21).
The clinical success rate of PTC with balloon dilation as a first line treatment is from 66% to 87% (Table 4) (30-35).

Full table
Bonnel et al. [2012] performed PTBD procedures with a balloon dilatation on 87 patients with postoperative bilio-enteric stricture. The procedures were successful in 74 out of the 87 patients treated, with a success rate of 85% (34). Janssen et al. [2014] preformed PTBD procedures with a balloon dilatation on 98 patients with postoperative bilioenteric stricture. The procedures were successful in 85 out of the 98 patients treated, with a success rate of 87% (35). DePietro et al. [2015] preformed PTBD procedures with a balloon dilatation on 71 patients with postoperative bilioenteric stricture. The procedures were successful in 46 out of the 71 patients treated, with a success rate of 65% (32).
Major complications (2%) are hemobilia, biliary loss, sepsis, cholangitis and pancreatitis. Stent placement may be associated with complications such as occlusion and distal (5.9%) or proximal (4.9%) migration (8,16,17,36).
The choice of the biliary stent (plastic or metallic) depends on the nature of the obstruction (benign vs. malignant) (17). Plastic biliary stents are made of polyethylene, polyurethane, or Teflon. The size of plastic stents is limited to 12Fr when placed endoscopically, or to 14Fr when placed percutaneously, whereby the created large intrahepatic tract can be challenging to seal.
Plastic internal stents are cheapest but reportedly prone to migration. Self-expanding metal stents (SEMS) are larger than plastic to reduce the possibility of migration and maintain patency longer, but are associated with increased risk of cholangitis (37). Mean patency rate of SEMS is 6–9 months with an occlusion rate of 30–40% (17). Metal stent are composed of metal alloys, such as platinum, nitinol, or stainless steel. The length of available SEMS ranges from 4 to 12 cm, and expanded diameters reach 6 to 10 mm.
SEMS can be fully-covered, partially covered, or uncovered (37). Bare or uncovered stent are composed by a mesh structure. This mesh permits to the endoprosthesis to be anchored into the biliary wall and diminishes the risk of migration. However, migration is reported in 20–50% of the cases within the next six months; moreover the neoplastic tissue can grow through the mesh and close the stent (38).
Recently, a new biodegradable stent in polydioxanone is used to treat benign biliary strictures with good results in terms of patency of the biliary stricture. The monofilament loses 50% of its breaking strength after three weeks and is absorbed within 6 months (39). Percutaneous balloon dilatation and stenting form the backbone of treatment for strictures of biliary enteric anastomoses. Multiple procedures are often required, over a period of several months after the initial operation, to achieve good long-term patency. Nevertheless, a small part of the patients require surgical revision of their anastomoses (3,29).
Mini invasive radiological techniques became the first-approach treatment. The possibility of quick access to interventional radiology specialist has been shown to be successful in the urgent management of postoperative complications and has reduced re-operations associated with high rates of morbidity and mortality (6-8).
Intraabdominal abscess
Intraabdominal abscess is defined as an infected fluid collection; it is reported in 5.0% to 8.0% of patients following pancreatic surgery (Table 5) (6,7). Several studies were conducted in order to establish the risk factors related to the formation of intraabdominal abscesses. The type of pancreatic-digestive anastomosis does not influence the appearance of this complication. Another paper showed that the early removal of intraoperative drainage, on the fourth day after surgery, allowed a reduction in intra-abdominal abscess rates compared to removal after the eighth day postoperative (40).

Full table
Intraabdominal abscesses can be superinfected through contamination by colonized bile and therefore must be treated in order to prevent other comorbidities such as abdominal haemorrhage, prolonged hospitalization, and higher mortality (40,41). The common signs and symptoms are abdominal pain (83%), fever (80.5%), chills and perspiration (56%). Undrained abdominal abscess has a high mortality rate between 45% and 100% (2).
In recent years, intraabdominal abscess management and outcomes have improved due to innovation on new image-guided drainage techniques and modern surgical procedures. Imaging-guided techniques of percutaneous abscess drainage (PAD) lead to minimal tissue trauma and low morbidity and mortality rates (40). PAD are considered the first line treatment in both the paediatric and adult patients, in the absence of indications for immediate surgery (2,41-43). Ultrasound, CT, and fluoroscopy are the imaging modalities most frequently used for PAD. The choice of image guidance is individualized for each case based on abscess location, patient factors, operator experience and preference, and equipment availability (42-44). Ultrasound guidance is a real-time imaging technique and it is the method of choice in superficial abscesses, where there is a low risk of damage vascular structures, bowel, or pleural cavity (2).
CT is the method of choice when US guidance does not seem safe enough (40). Contraindications to percutaneous catheter placement include an uncorrected coagulopathy, lack of a safe percutaneous access route and inability of the patient to cooperate (2,42,44).
PAD may be performed using the Seldinger technique or the trocar technique, after subcutaneous administration of anaesthesia and IV moderate sedation. The Seldinger technique is a multistep procedure in which dilation and drain placement are performed after proper needle placement (2,41,42,44,45). The trocar technique is a monophasic procedure that is performed with a coaxial needle and drainage system. The trocar method avoids the risk of losing guidewire access during dilator exchanges performed in the Seldinger technique (42,44,46). The trocar technique may be preferable when crossing resistant structures or when Seldinger technique would increase patient discomfort (44).
According to the Society of Interventional Radiology guidelines, major complications may include: catheter-related pain, haemorrhage, infection and sepsis, fistula formation or visceral injury along the access route such as bowel obstruction or perforation (2,41). Indeed, minor complications are referring to catheter dislodgement and obstruction (41). The complication rate shown in literature is 2.5% (41). When PAD fails for the presence of septations or highly viscous contents, thrombolytic agents may be used to facilitate complete evacuation of abscess contents such as intracavitary urokinase in adults, tissue type plasminogen activator in paediatric patients (42-44).
Catheter removal may be considered when the patient is clinically improved and no longer feverish, and catheter output has decreased to <10–15 mL/day. In univariate and multivariate analyses, three independent variables were associated with a significantly lower rate of primary success of inserted PAD (45). These risks were: predisposing pathological conditions (risk 2.4 times higher than patients with other causes of abscess), presence of multiple abscesses (risk 2.5 times higher than patients with a single abscess), drainage removal with continuous daily production ≥15 mL/d (risk 4.1 times higher than those with lower drainage drain) (45). Success rate for CT-guided PAD ranges from 75.7–86.9% and 100%, with only a few patients requiring new operative drainage (5,6,8,13,43,47).
Sohn et al. [2003] preformed PAD procedures on 84 patients with postoperative intraabdominal abscesses. The procedures were successful in 73 out of the 84 patients treated, with a success rate of 87% (13). Carrafiello et al. [2011] preformed PAD procedures on 11 patients with postoperative intraabdominal abscesses. The procedures were successful in 11 out of the 11 patients treated, with a success rate of 100% (47). Casadei et al. [2014] preformed PAD procedures on 37 patients with postoperative intraabdominal abscesses. The procedures were successful in 28 out of the 37 patients treated, with a success rate of 76% (Table 6) (5).

Full table
Conclusions
Advances in surgical technique and perioperative management of pancreatic surgeries have decreased postsurgical mortality; however, postoperative morbidity of patients remains 20–60% due to the complexity of these procedures (3,6,7,14). Transient jaundice and cholangitis are biliary complications that lead to a favorable outcome without invasive interventions, in contrast to bilio-enteric stricture, biliary leaks and intra-abdominal abscesses that risk further radiological or surgical treatments (3,4,11,13).
Surgical re-exploration is difficult and requires a high surgical competence due to the presence of oedema and scar tissue. Interventional radiology procedures are preferred as first line therapeutic option to avoid more invasive procedures (surgical or endoscopic) (47).
The role of the interventional radiologist is to diagnose the biliary damage and to treat with a definitive curative purpose or as a bridge for re-intervention. IR is an expanding field for the treatment of post-surgical complications that prevent the morbidity of re-intervention and long periods of hospitalization. A wide range of IR treatment options are currently available with a pivotal role in patient management. PAD, PTC, and PTBD are safe and effective procedures for the treatment of postsurgical biliary duct injuries (5,11).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Shahnazi M, Khatami A, Jamzad A, et al. Safety and Efficacy of Percutaneous CT-Guided Drainage in the Management of Abdominopelvic Abscess. Iran J Radiol 2014;11:e20876. [Crossref] [PubMed]
- Kadaba RS, Bowers KA, Khorsandi S, et al. Complications of biliary-enteric anastomoses. Ann R Coll Surg Engl 2017;99:210-5. [Crossref] [PubMed]
- Malgras B, Duron S, Gaujoux S, et al. Early biliary complications following pancreaticoduodenectomy: prevalence and risk factors. HPB (Oxford) 2016;18:367-74. [Crossref] [PubMed]
- Casadei R, Ricci C, Giampalma E, et al. Interventional radiology procedures after pancreatic resections for pancreatic and periampullary diseases. JOP 2014;15:378-82. [PubMed]
- Baker TA, Aaron JM, Borge M, et al. Role of interventional radiology in the management of complications after pancreaticoduodenectomy. Am J Surg 2008;195:386-90; discussion 390. [Crossref] [PubMed]
- Sanjay P, Kellner M, Tait IS. The role of interventional radiology in the management of surgical complications after pancreatoduodenectomy. HPB (Oxford) 2012;14:812-7. [Crossref] [PubMed]
- Tejedor L, Tejedor L, Serrablo A. Postoperative Pancreatic Biliary Surgical Complications. J Gastroenterol Hepatol Res 2013;2:661-71.
- Raman SP, Horton KM, Cameron JL, et al. CT After Pancreaticoduodenectomy: Spectrum of Normal Findings and Complications. AJR Am J Roentgenol 2013;201:2-13. [Crossref] [PubMed]
- Slanetz PJ, Boland GW, Mueller PR. Imaging and interventional radiology in laparoscopic injuries to the gallbladder and biliary system. Radiology 1996;201:595-603. [Crossref] [PubMed]
- May K, Hunold P. Leakage of Hepaticojejunal Anastomosis: Radiological Interventional Therapy. Visc Med 2017;33:192-6. [Crossref] [PubMed]
- Antolovic D, Koch M, Galindo L, et al. Hepaticojejunostomy-analysis of risk factors for postoperative bile leaks and surgical complications. J Gastrointest Surg 2007;11:555-61. [Crossref] [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Pancreaticoduodenectomy Role of Interventional Radiologists in Managing Patients and Complications. J Gastrointest Surg 2003;7:209-19. [Crossref] [PubMed]
- DeOliveira ML, Winter JM, Schafer M, et al. Assessment of Complications After Pancreatic Surgery. Ann Surg 2006;244:931-7. [Crossref] [PubMed]
- Duconseil P, Turrini O, Ewald J, et al. Biliary Complications After Pancreaticoduodenectomy: Skinny Bile Ducts Are Surgeons’ Enemies. World J Surg 2014;38:2946-51. [Crossref] [PubMed]
- Saad WE, Wallace MJ, Wojak JC, et al. Quality Improvement Guidelines for Percutaneous Transhepatic Cholangiography, Biliary Drainage, and Percutaneous Cholecystostomy. J Vasc Interv Radiol 2010;21:789-95. [Crossref] [PubMed]
- Irani S, Baron TH, Akbar A, et al. Endoscopic Treatment of Benign Biliary Strictures Using Covered Self-Expandable Metal Stents (CSEMS). Dig Dis Sci 2014;59:152-60. [Crossref] [PubMed]
- Park JH, Oh JH, Yoon Y, et al. Acetic Acid Sclerotherapy for Treatment of a Biliary Leak from an Isolated Bile Duct after Hepatic Surgery. J Vasc Interv Radiol 2005;16:885-8. [Crossref] [PubMed]
- Carrafiello G, Ierardi AM, Piacentino F, et al. Percutaneous transhepatic embolization of biliary leakage with N-butyl cyanoacrylate. Indian J Radiol Imaging 2012;22:19-22. [Crossref] [PubMed]
- Ierardi AM, Fontana F, Mangini M, et al. Use of Amplatzer Vascular Plug to Treat a Biliary Cutaneous Fistula. Korean J Radiol 2013;14:801. [Crossref] [PubMed]
- Fidelman N. Benign Biliary Strictures: Diagnostic Evaluation and Approaches to Percutaneous Treatment. Tech Vasc Interv Radiol 2015;18:210-7. [Crossref] [PubMed]
- Popat B, Thakkar D, Deshmukh H, et al. Percutaneous Transhepatic Biliary Drainage in the Management of Post-surgical Biliary Leaks. Indian J Surg 2017;79:24-8. [Crossref] [PubMed]
- Cozzi G, Severini A, Civelli E, et al. Percutaneous Transhepatic Biliary Drainage in the Management of Postsurgical Biliary Leaks in Patients with Nondilated Intrahepatic Bile Ducts. Cardiovasc Intervent Radiol 2006;29:380-8. [Crossref] [PubMed]
- Mastier C, Valette PJ, Adham M, et al. Complex Biliary Leaks: Effectiveness of Percutaneous Radiological Treatment Compared to Simple Leaks in 101 Patients. Cardiovasc Intervent Radiol 2018;41:1566-72. [Crossref] [PubMed]
- Pedicini V, Poretti D, Mauri G, et al. Management of post-surgical biliary leakage with percutaneous transhepatic biliary drainage (PTBD) and occlusion balloon (OB) in patients without dilatation of the biliary tree: preliminary results. Eur Radiol 2010;20:1061-8. [Crossref] [PubMed]
- Aytekin C, Boyvat F, Harman A, et al. Percutaneous management of anastomotic bile leaks following liver transplantation. Diagn Interv Radiol 2007;13:101-4. [PubMed]
- Righi D, Franchello A, Ricchiuti A, et al. Safety and efficacy of the percutaneous treatment of bile leaks in hepaticojejunostomy or split-liver transplantation without dilatation of the biliary tree. Liver Transpl 2008;14:611-5. [Crossref] [PubMed]
- Stampfl U, Hackert T, Radeleff B, et al. Percutaneous Management of Postoperative Bile Leaks After Upper Gastrointestinal Surgery. Cardiovasc Intervent Radiol 2011;34:808-15. [Crossref] [PubMed]
- House MG, Cameron JL, Schulick RD, et al. Incidence and Outcome of Biliary Strictures After Pancreaticoduodenectomy. Ann Surg 2006;243:571-6; discussion 576-8. [Crossref] [PubMed]
- Lee AY, Gregorius J, Kerlan RK, et al. Percutaneous Transhepatic Balloon Dilation of Biliary-Enteric Anastomotic Strictures after Surgical Repair of Iatrogenic Bile Duct Injuries. Mandell MS, editor. PLoS One 2012;7:e46478.
- Köcher M, Černá M, Havlík R, et al. Percutaneous treatment of benign bile duct strictures. Eur J Radiol 2007;62:170-4. [Crossref] [PubMed]
- DePietro DM, Shlansky-Goldberg RD, Soulen MC, et al. Long-Term Outcomes of a Benign Biliary Stricture Protocol. J Vasc Interv Radiol 2015;26:1032-9. [Crossref] [PubMed]
- Glas L, Courbière M, Ficarelli S, et al. Long-term Outcome of Percutaneous Transhepatic Therapy for Benign Bilioenteric Anastomotic Strictures. J Vasc Interv Radiol 2008;19:1336-43. [Crossref] [PubMed]
- Bonnel DH, Fingerhut AL. Percutaneous transhepatic balloon dilatation of benign bilioenteric strictures: Long-Term results in 110 patients. Am J Surg 2012;203:675-83. [Crossref] [PubMed]
- Janssen JJ, van Delden OM, van Lienden KP, et al. Percutaneous Balloon Dilatation and Long-Term Drainage as Treatment of Anastomotic and Nonanastomotic Benign Biliary Strictures. Cardiovasc Intervent Radiol 2014;37:1559-67. [Crossref] [PubMed]
- Hair CD, Sejpal DV. Future developments in biliary stenting. Clin Exp Gastroenterol 2013;6:91-9. [PubMed]
- Moy BT, Birk JW. An Update to Hepatobiliary Stents. J Clin Transl Hepatol 2015;3:67-77. [Crossref] [PubMed]
- Tsetis D, Krokidis M, Negru D, et al. Malignant biliary obstruction: the current role of interventional radiology. Ann Gastroenterol 2016;29:33-6. [PubMed]
- Giménez ME, Palermo M, Houghton E, et al. Biodegradable Biliary Stents: A New Approach For The Management Of Hepaticojejunostomy Strictures Following Bile Duct Injury. Prospective Study. Arq Bras Cir Dig. 2016;29:112-6. [Crossref] [PubMed]
- Lermite E, Sommacale D, Piardi T, et al. Complications after pancreatic resection: Diagnosis, prevention and management. Clin Res Hepatol Gastroenterol 2013;37:230-9. [Crossref] [PubMed]
- Zhao N, Li Q, Cui J, et al. CT-guided special approaches of drainage for intraabdominal and pelvic abscesses. Medicine 2018;97:e12905. [Crossref] [PubMed]
- Arani K, Nandalur K, Tucker CM, et al. Image-guided Percutaneous Drainage in the Pediatric Population: A Primer for Radiologists. J Clin Imaging Sci 2011;1:31. [Crossref] [PubMed]
- Asai N, Ohkuni Y, Yamazaki I, et al. Therapeutic impact of CT-guided percutaneous catheter drainage in treatment of deep tissue abscesses. Braz J Infect Dis 2013;17:483-6. [Crossref] [PubMed]
- Brown C, Kang L, Kim ST. Percutaneous drainage of abdominal and pelvic abscesses in children. Semin Intervent Radiol 2012;29:286-94. [Crossref] [PubMed]
- Haider SJ, Tarulli M, McNulty NJ, et al. Liver Abscesses: Factors That Influence Outcome of Percutaneous Drainage. AJR Am J Roentgenol 2017;209:205-13. [Crossref] [PubMed]
- Turan HG, Özdemir M, Acu R, et al. Comparison of seldinger and trocar techniques in the percutaneous treatment of hydatid cysts. World J Radiol 2017;9:405-12. [Crossref] [PubMed]
- Carrafiello G, Laganà D, Dizonno M, et al. Emergency percutaneous treatment in surgical bile duct injury. Emerg Radiol 2008;15:335-41. [Crossref] [PubMed]