
Role of computed tomography and magnetic resonance imaging in local complications of acute pancreatitis
Introduction
Acute pancreatitis (AP) represents a pancreas inflammation of sudden onset; it can occur with different degrees of severity ranging from mild gland inflammation to massive pancreatic necrosis. The annual incidence of acute pancreatitis varies from 5 to 70 new cases per 100,000 people and, in the USA is responsible for over 200,000 hospital admissions each year (1-3). Biliary calculosis and alcohol abuse cause about 70% of cases of acute pancreatitis (4). Less common causes are metabolic disorders (hypertriglyceridemia and hypercalcemia), drugs (azathioprine and mercaptopurine), infections (paramyxovirus, coxsackievirus, ascaris lumbricoides), tumours (pancreatic adenocarcinoma and lymphoma), abdominal trauma (especially in children), endoscopic retrograde cholangiopancreatography (ERCP), functional alteration of the sphincter of Oddi and congenital anomalies (coledococele, pancreas divisum and duodenal duplication cyst). Furthermore, twenty percent of cases of acute pancreatitis are idiopathic, although most are thought to be caused by the passage of biliary sludges and microlithiasis (4,5). Acute pancreatitis can simulate other clinical disorders like inferior wall myocardial infarction and other causes of acute abdomen (intestinal obstruction, mesenteric ischemia or infarction, perforation of gastric or duodenal ulcer, biliary colic and aortic dissection) (6). According to the recent guidelines, to diagnose this condition it is necessary fulfilling two of the following three criteria (7): (I) epigastric pain, more or less intense, often radiating to the back and generally associated with nausea and vomiting; (II) increase of serum amylase and lipase values at least three times compared to normal limits; (III) characteristic findings of acute pancreatitis on contrast-enhanced computed tomography (CT) and less commonly magnetic resonance imaging (MRI) or ultrasound (US). Diagnosis of acute pancreatitis can be confirmed even without the use of radiological imaging. CT or MRI may be required to identify the cause, to assess the degree of severity, to predict the course and to identify possible complications.
Severity level of acute pancreatitis
AP severity assessment is one of the most important issue in disease management. Atlanta Classification was formulated in 1992 in the attempt to classify acute pancreatitis and its complications (8). This classification was modified and implemented in 2012 and 2016. The revised Atlanta classification subdivides acute pancreatitis into two types: interstitial edematous pancreatitis (IEP) and necrotizing pancreatitis. These changes have allowed both to improve the differences between acute IEP and acute necrotising pancreatitis, and to implement the definitions of its complications. The clinical evaluation of severe AP is often complex and unreliable; it is estimated that even experienced doctors in less than half of the cases (40%) will be able to predict which patients will develop severe AP based only on clinic results (9). The most used clinical scores developed with the aim of more accurately and reproducibly assessing the severity of AP are the APACHE II criteria, Ranson criteria and Glasgow criteria (10).
When and how to perform CT and MRI
In most cases the symptoms of AP are nonspecific and, as reported in the literature, serum lipase and amylase levels do not correlate with the severity of the disease. Contrast-enhanced CT (using iodinated contrast medium injected intravenously at a flow rate of 3–5 mL/sec) is recommended when is necessary to confirm the diagnosis, identify (where possible) cause and complications, rule out alternative causes of abdominal pain, assess the extent of acute pancreatitis and also for the preoperative planning (1,11).
CT acquisition protocol consists of:
- unenhanced acquisition, if possible preceded by the oral administration of 500 mL of water acting as negative contrast increases the difference between the second duodenal portion and the head of the pancreas. This phase allows also the identification of some causes of acute pancreatitis (e.g., biliary microlithiasis);
- parenchymal phase (40 seconds) is the optimal phase for the identification of pancreatic necrosis areas. In fact, in this phase the healthy pancreatic tissue has the maximum enhancement;
- portal phase (70–80 seconds) extended to the whole abdomen useful for identifying some complications (e.g., venous thrombosis) and associated pathologies.
To these phases we can add the arterial phase (20 seconds) and a delayed phase (3–5 minutes) for the detection of haemorrhage and pseudoaneurysms. It is common to use a double-phase technique (parenchymal and portal phases) but with this protocol we risk missing the haemorrhagic collections (11). MRI is comparable to CT for the diagnosis of acute pancreatitis but requires much more time so it is not usually chosen in the emergency scenario. In clinical practice we use MRI when the patient is allergic to iodine contrast, when we want to better evaluate the pancreatic ductal system and biliary three, the bile duct and also for a better characterization of peri-pancreatic collections (12). CT is considered the gold standard in patients with AP; however, it exposes patients to radiation burden, increased by follow-up examinations; furthermore, the use of iodinated contrast media can potentially aggravate acute pancreatitis (13).
Advantaged of MRI use are:
- MRI is a diagnostic imaging method with no radiation hazard, which might be suitable for patients with multiple follow-up controls;
- MRI has fewer contraindications than CT and is a reliable method for severity staging of acute pancreatitis, which has predictive value for the prognosis of the disease;
- MRI cholangiopancreatography (MRCP) has the unique capability of providing non-invasive images of the pancreatic ducts and can demonstrate possible communication of a pancreatic pseudocyst with pancreatic ducts (14);
- MRI is useful for assessing signal intensity of fluid exudation or pseudocysts; to identify local haemorrhage or pseudoaneurysm, which might help plan the surgery.
Regarding the MRI protocol today, the introduction of the fat-suppression techniques, breath-hold fast sequences and phased-array coils has permitted to increase contrast resolution of pancreatic and peripancreatic tissues.
Consequently, the use of MRI has become more frequent in patients with AP complications. The acquisition protocol requires the combined use of T1-weighted (T1-w), T2-weighted sequence (T2-w), MRCP sequences and T1-w sequences with fat suppression [e.g., fast spin-echo (FSE)] imaging with multiple breath-hold acquisitions or single-breath-hold gradient echo imaging to improve the delineation of pancreatic borders and the pancreas itself; T1-w sequences allow also the evaluation of haemorrhagic complications of acute pancreatitis (15-17). T2-w sequences [e.g., fast recovery fast spin-echo (FRFSE) triggered or breath-hold single-shot fast spin-echo (SSFSE)] imaging has significant advantages in demonstrating fluid-filled lesions in or around the pancreas and the pancreatic duct (17,18). SSFSE T2-w sequences can be used to guide acquisition of an MRCP series which (obtained before gadolinium administration) allows non-invasive evaluation of pancreatic ducts and the whole extrahepatic biliary tract, and provides few respiratory artefacts or susceptibility effects (17,19). Diffusion weighted imaging (DWI) can display the manifestations of AP with water molecules restriction in an earlier phase compared to other imaging modalities and without radiation hazard (20).
Dynamic imaging after intravenous administration of gadolinium performed with T1-w acquisition [e.g., liver acquisition with volume acceleration (LAVA)] with the same timing of contrast-enhanced CT gives a comprehensive evaluation of the extent of the necrosis and the full range of the inflammatory extension for the initial staging of acute pancreatitis. Moreover, MR angiography, the post-processing technique after MR LAVA, can be performed to supplement the information for visualization of pancreatic vascular network and vascular complications of acute pancreatitis (17,19).
Different forms of pancreatitis
IEP
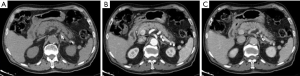
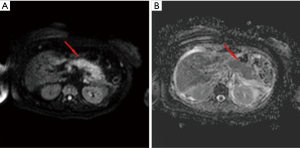
IEP is the most common type of AP, found in 90–95% of cases. CT detects a focal or diffuse parenchymal enlargement caused by inflammatory oedema and, after contrast administration of contrast medium, the pancreatic parenchyma will show a homogeneous enhancement. In addition to this, generally the peripancreatic fat will show fluid imbibition, which is often associated to peripancreatic effusion (Figure 1). MRI shows an increased signal of the pancreas and peripancreatic tissues in T2-w sequences, a low signal in T1-w sequences in the same locations, and restriction of the water molecules on DWI (Figure 2). Clinical symptoms of interstitial pancreatitis usually resolve within the first week (21,22).
Necrotizing pancreatitis
Necrotizing pancreatitis occurs in 5–10% of cases; it is characterized by a protracted clinical course, a high incidence of local complications, and a high mortality rate.
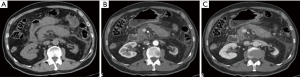
There are three subtypes of necrotizing pancreatitis; the subtypes are based on the anatomic area of necrotic involvement: (I) pancreatic only; (II) peripancreatic only; and (III) combined pancreatic and peripancreatic. The latter subtype is the most common (75% of cases). In this type of pancreatitis one or more areas of pancreatic parenchyma or peripancreatic tissue show an unenhanced or minimally enhanced (<30 HU) areas on contrast-enhanced CT or gadolinium enhanced MR images (10,19,23) (Figure 3). This imaging is established within a few days, this explains why an early CT can underestimate the extent of necrosis (23-26). The evolution of pancreatic necrosis is variable, can remain solid or liquefy, remain sterile or become infected, disappear or persist over time. These patients have higher morbidity than patients with IEP.
Complications
Complications of AP can be distinguished in localized and generalized. Among the localized complications we can identify: acute peripancreatic fluid collections (APFC), pseudocysts, acute necrotic collections (ANC), walled off pancreatic necrosis (WOPN), venous thrombosis, pseudoaneurysms and haemorrhage. The old term pancreatic abscess is abandoned because any collection can be sterile or infected, otherwise the infection occurs more often in the necrotic collections (27). Multiple organ failure syndrome (MOFS) and sepsis are possible generalized complications of AP.
APFC and pseudocyst
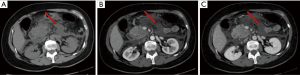
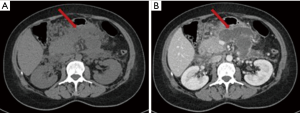
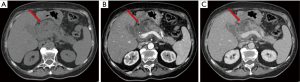
The acute peripancreatic fluid collection represents an early complication (<4 weeks) of acute IEP. APFCs can occur in the first hours after the onset of symptoms and are composed exclusively of fluid material. Contrast-enhanced CT will show fluid, capsule-free collections arranged around the pancreas (Figure 4). MRI, with fat suppressed T1- and T2-w sequences, can accurately depict APFCs with liquid signal performance of hypointensity on T1-w images and hyperintensity on T2-w images (Figure 5) (16). In some cases, APFCs will be placed in the anterior pararenal space (more commonly on the left), transverse mesocolon, mesenteric root, gastro-hepatic ligaments, gastrosplenic and gastrocolic (18,28,29). APFCs remain sterile and disappear spontaneously within 2–4 weeks in 50% of patients. If APFCs are sterile, it is not appropriate to drain them because they usually resolve spontaneously and their aspiration may cause infection (30). Only infected APFCs have to be drained. If an APFC did not resolve after 4 weeks, it becomes more organized and develops a fibrous tissue capsule. This collection is called pseudocyst and represents the late complication (>4 weeks) of IEP. Pseudocysts develop in less than 10% of IEP cases (18). According to spatial locations, pancreatic pseudocysts are classified as intraparenchymal or extrapancreatic. The intraparenchymal pseudocyst might be communicating with pancreatic ducts and associated with partial pancreatic ductal obstruction (29,31). Among the most important complications of pseudocysts we can identify infection, compression on adjacent organs (stomach, duodenum, biliary system) and rupture (peritonitis). At contrast-enhanced CT the pseudocysts appear as collections of well-circumscribed peripancreatic fluids, usually round or oval of homogeneously low density, surrounded by a well-defined wall with enhancement (Figure 6). MR findings of a simple pseudocyst include a round or oval fluid collection surrounded by a thin wall, with liquid signal performance of hypointensity on T1-w images and hyperintensity on T2-w images (Figure 7). Pancreatic pseudocysts can also present as complex pseudocysts associated with mucus, protein, and haemorrhage with heterogeneous hyperintense signal on T1-w images with fat suppression (29,31). The three main treatment modalities are endoscopic drainage (the preferred treatment), percutaneous drainage and surgical drainage.
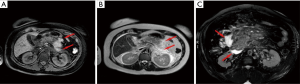
ANC and WOPN
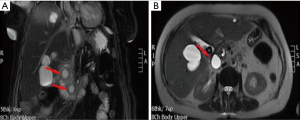
The ANCs represent an early complication (<4 weeks) of acute necrotizing pancreatitis. Unlike the APFC their content is heterogeneous and consists of fluid, solid debris and fat. They can be single or multiple and be in a peripancreatic position or spread throughout the abdomen. The contrast-enhanced CT will allow us to see heterogeneous, wall less collections in a patient with acute necrotic pancreatitis (Figure 8). MRI (with fat suppressed T1- and T2-w sequences) can accurately depict ANC with liquid signal performance of hypointensity on T1-w images and hyperintensity on T2-w images with areas of haemorrhage or necrosis of pancreas and peripancreatic fat tissue which appear with iso-hyperintensity on T1w images (Figures 9,10).
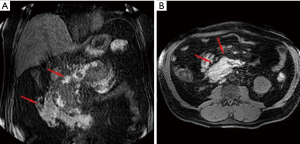
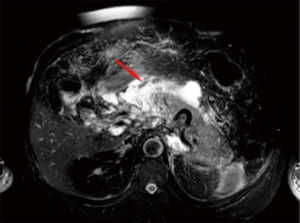
The contents of the collection can be sterile or infected. Sterile pancreatic necrosis does not require treatment, especially if the patient has a stable clinical status. However, the possibility of infection increases with the time. After 4 weeks the acute necrotic collection will develop a thick capsule visible at contrast-enhanced CT. This formation will be called WOPN and represents the late complication (>4 weeks) of acute necrotizing pancreatitis. WOPN is an irregular, partially liquefied collection, which may contain solid and fat debris (Figure 11). MR T1- and T2-w images with and without fat suppression, can easily and better than CT, depict necrotic debris or haemorrhagic components of the pancreatic parenchyma with signal hyperintensity on T1-w sequences and hypointensity on T2-w sequences (Figure 10) (15).
WOPN can be sterile or infected. The differentiation of WOPN from the pancreatic pseudocyst is essential because management differs. WOPN may need aggressive treatment (most centres prefer the treatment with operative necrosectomy in the infected or symptomatic cases) to avoid complications.
Venous thrombosis
Splanchnic venous thrombosis is a rare complication of AP. Venous thrombosis often involves the splenic vein, the portal vein and the superior mesenteric vein, both in combination or separately.
Splenic vein thrombosis is the most common form and is due to the inflammatory intimal injury or to the ab-extrinsic compression by the fluid collections (32). This can cause portal hypertension, development of venous ectasia and splenic infarction (33).
Pseudoaneurysm
Pseudoaneurysm is a rare but serious complication of acute pancreatitis and occurs in 4% to 10% of cases (34). Erosion of the arteries is caused by the proteolytic enzymes released by the pancreas (35-37). Pseudoaneurysms can break in the peritoneal cavity, in the retroperitoneum, in adjacent collections and rarely in the pancreatic duct (35). For their diagnosis it is useful to perform CT in the arterial phase.
Haemorrhage
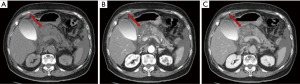
Like pseudoaneurysms, haemorrhage is caused by the release of proteolytic enzymes from the pancreas. Fortunately, it is a rare complication but it is often lethal. Splenic artery, gastroduodenal artery and pancreatic-duodenal artery are the most frequent involved arteries (38). In this case it is useful to perform CT in the arterial phase (Figure 12). T1-w fat suppression images may reveal the presence of bleeding prior contrast agent administration.
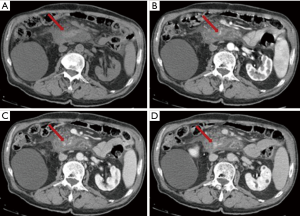
Abdominal compartment syndrome
Abdominal compartment syndrome can be defined as an “acute elevation of the intraabdominal pressure with organ dysfunction”. Haemorrhagic pancreatitis and large amount of pancreatic ascites can be one of the causes of abdominal compartment syndrome. The prevalence of intraabdominal hypertension in patients with severe acute pancreatitis is about 40–50% (39). Diagnosis of abdominal compartment syndrome is often complicated; multiorgan failure, sepsis and acute respiratory distress syndrome are often seen in these patients. Radiological findings are few and non-specific. Between the radiological findings that can be found in these patients we can describe: elevated diaphragm, rounded configuration of abdominal wall (anteroposterior-to-lateral girth ratio >0.8), hemoperitoneum, flattened inferior vena cava, flattened renal veins, mosaic liver perfusion, increased bowel enhancement, increased gastric wall enhancement, gastric distention, reduced diastolic flow in portal, hepatic or renal veins on sonography (40).
Conclusions
Acute pancreatitis represents a frequent cause of acute abdomen and its complications are still a cause of death. CT and MRI represent the best clinical and surgery friend in the identification of acute pancreatitis and its complications. Imaging findings of acute pancreatitis are crucial for the timing and management of acute pancreatic complications in the emergency setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Merkle EM, Görich J. Imaging of acute pancreatitis. Eur Radiol 2002;12:1979-92. [Crossref] [PubMed]
- Frey CF, Zhou H, Harvey DJ, et al. The incidence and case fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994-2001. Pancreas 2006;33:336-44. [Crossref] [PubMed]
- Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006;101:2379-400. [Crossref] [PubMed]
- Spanier BW, Dijkgraaf MG, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: an update. Best Pract Res Clin Gastroenterol 2008;22:45-63. [Crossref] [PubMed]
- Ranson JH. Acute pancreatitis: pathogenesis, outcome and treatment. Clin Gastroenterol 1984;13:843-63. [PubMed]
- Kwon RS, Banks PA. How should acute pancreatitis be diagnosed in clinical practice? In: Dominguez-Munõz JE, Malfertheiner P. editors. Clinical pancreatology for practicing gastroenterologists and surgeons. Malden, MA: Blackwell, 2005;4:34-9.
- Banks PA, Bollen TL, Dervenis C, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Bradley EL III. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993;128:586-90. [Crossref] [PubMed]
- Wilson C, Heath DI, Imrie CW. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg 1990;77:1260-4. [Crossref] [PubMed]
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002;223:603-13. [Crossref] [PubMed]
- Urban BA, Fishman EK. Tailored helical CT evaluation of acute abdomen. RadioGraphics 2000;20:725-49. [Crossref] [PubMed]
- Hirota M, Kimura Y, Ishiko T, et al. Visualization of the heterogeneous internal structure of so-called “pancreatic necrosis” by magnetic resonance imaging in acute necrotizing pancreatitis. Pancreas 2002;25:63-7. [Crossref] [PubMed]
- McMenamin DA, Gates LK Jr. A retrospective analysis of the effect of contrast-enhanced CT on the outcome of acute pancreatitis. Am J Gastroenterol 1996;91:1384-7. [PubMed]
- Gillams AR, Kurzawinski T, Lees WR. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol 2006;186:499-506. [Crossref] [PubMed]
- Arvanitakis M, Delhaye M, De Maertelaere V, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology 2004;126:715-23. [Crossref] [PubMed]
- Xiao B, Zhang XM. Magnetic resonance imaging for acute pancreatitis. World J Radiol 2010;2:298-308. [Crossref] [PubMed]
- Matos C, Cappeliez O, Winant C, et al. MR imaging of the pancreas: a pictorial tour. Radiographics 2002;22:e2. [Crossref] [PubMed]
- Lenhart DK, Balthazar EJ. MDCT of acute mild (nonnecrotizing) pancreatitis: abdominal complications and fate of fluid collections. AJR Am J Roentgenol 2008;190:643-9. [Crossref] [PubMed]
- Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology 1994;193:297-306. [Crossref] [PubMed]
- Thomas S, Kayhan A, Lakadamyali H, et al. Diffusion MRI of acute pancreatitis and comparison with normal individuals using ADC values. Emergency Radiology 2012;19:5-9. [Crossref] [PubMed]
- Morgan DE. Imaging of acute pancreatitis and its complications. Clin Gastroenterol Hepatol 2008;6:1077-85. [Crossref] [PubMed]
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011;9:1098-103. [Crossref] [PubMed]
- Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331-6. [Crossref] [PubMed]
- Spanier BWM, Nio Y, van der Hulst RWN, et al. Practice and yield of early CT scan in acute pancreatitis: a Dutch Observational Multicenter Study. Pancreatology 2010;10:222-8. [Crossref] [PubMed]
- Bollen TL, Singh VK, Maurer R, et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity of acute pancreatitis. Am J Gastroenterol 2012;107:612-19. [Crossref] [PubMed]
- Isenmann R, Buechler M, Uhl W, et al. Pancreatic necrosis: an early finding in severe acute pancreatitis. Pancreas 1993;8:358-61. [Crossref] [PubMed]
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Balthazar EJ. Complications of acute pancreatitis: clinical and CT evaluation. Radiol Clin North Am 2002;40:1211-27. [Crossref] [PubMed]
- Andrén-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part I: classification, pathophysiology, anatomic considerations and treatment. JOP 2004;5:8-24. [PubMed]
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012;262:751-64. [Crossref] [PubMed]
- Kim YH, Saini S, Sahani D, et al. Imaging diagnosis of cystic pancreatic lesions: pseudocyst versus nonpseudocyst. Radiographics 2005;25:671-85. [Crossref] [PubMed]
- Butler JR, Eckert GJ, Zyromski NJ, et al. Natural history of pancreatitis-induced splenic vein thrombosis: a systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford) 2011;13:839-45. [Crossref] [PubMed]
- Mortelé KJ, Mergo PJ, Taylor HM, et al. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast enhanced helical CT findings. Eur J Radiol 2004;52:67-72. [Crossref] [PubMed]
- Bergert H, Hinterseher I, Kersting S, et al. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery 2005;137:323-28. [Crossref] [PubMed]
- Kim JH, Kim JS, Kim CD, et al. Clinical features of pseudoaneurysms complicating pancreatitis: single center experience and review of Korean literature. Korean J Gastroenterol 2007;50:108-15. [PubMed]
- Araki K, Shimura T, Watanabe A, et al. Gastric bleeding from a penetrating pancreatic pseudocyst with pseudoaneurysm of the splenic artery. Hepatogastroenterology 2009;56:1411-3. [PubMed]
- Cahow CE, Gusberg RJ, Gottlieb LJ. Gastrointestinal hemorrhage from pseudoaneurysms in pancreatic pseudocysts. Am J Surg 1983;145:534-41. [Crossref] [PubMed]
- Memiş A, Parildar M. Interventional radiological treatment in complications of pancreatitis. Eur J Radiol 2002;43:219-28. [Crossref] [PubMed]
- De Waele JJ, Hesse UJ. Life saving abdominal decompression in a patient with severe acute pancreatitis. Acta Chir Belg 2005;105:96-8. [Crossref] [PubMed]
- Patel A, Lall CG, Jennings SG, et al. Abdominal compartment syndrome. AJR Am J Roentgenol 2007;189:1037-43. [Crossref] [PubMed]


