
Approach to cytological indeterminate thyroid nodules
Introduction
Indeterminate thyroid nodules on cytology represent a challenge for both clinicians and pathologists. Clinicians consider all thyroid fine-needle aspiration results that do not give a definitive (clearly benign or clearly malignant) result as “indeterminate”. Moreover, the new WHO classification of endocrine organs introduced a new concept in encapsulated follicular patterned lesions on histology (1). The absence, questionable and presence of vascular/capsular invasion and the absence, questionable and presence of papillary-like nuclear features also give rise to different entities according to the possible combination of these features. All these new entities do not carry the definition of carcinoma in their name anymore, but that of “Tumor of uncertain malignant potential” divided into “Follicular tumor of uncertain malignant potential (FT-UMP), “Well-differentiated tumor of uncertain malignant potential” (WDT-UMP) and “Non-invasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP), thus increasing the confusion (1). Pathologists are discordant as to what an “indeterminate” cytological diagnosis really represents. Some include the atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS), the follicular neoplasm or suspicious for a follicular neoplasm (FN/SFN) and the suspicious for malignancy (SM) diagnostic categories (DC) (or the THY3 and THY4 in the English classification) in this group, while others only include the AUS/FLUS and FN/SFN DC (or THY3) (2,3). In this review, we consider “indeterminate lesions” to be only those cases with an AUS/FLUS or FN/SFN (or THY3) diagnosis. The SM DC carries a risk of malignancy (ROM) in our hands of 60–75%, that justify the need for surgery and in our opinion does not allow a classification into “indeterminate lesion” (2). Epidemiology, ultrasonographic, cytological and molecular features useful in refining the ROM and the management of indeterminate cytological diagnosis will be reviewed in this article.
Epidemiology
The frequency of indeterminate nodules varies greatly in literature (4). For the AUS/FLUS DC, frequencies varying from 3% to 27.2% with an overall value of 9.6% have been reported, while cases in the FN/SFN DC ranged from 1.2% to 25.3% with an overall value of 10.1%. The overall incidence for indeterminate nodules is around 19.7% of all thyroid fine-needle aspiration cytology (FNAC). The prevalence of WDT-UMP and FT-UMP has been reported as having wide variations by the WHO blue book, 0.5% to 8.1% and 0.6% to 7.6%, respectively. For NIFTP, the reference paper states a prevalence of 18.6%, but lower values have been recorded in Canada (prevalence of 2.1%) and in Asia (2.9%) (1,5-7). In our opinion, the introduction of the NIFTP entity will cause a further increase in the incidence of indeterminate thyroid nodules in FNAC specimens in the future. We have already observed an increase of the overall incidence of thyroid cancers, especially for the small and indolent papillary microcarcinomas (8). Better access to health services and the advent of new diagnostic and more performant tools, primarily ultrasonography and imaging techniques, have been advocated as a major cause in increasing incidence (8). Clinically, we are now capable to detect smaller and even more deeply located lesions. In the United States, thyroid cancer incidence has increased by 3.6% per year on average, while incidence-based mortality increased 1.1% per year (9).
Cytological features
The cytological features of the indeterminate category can be roughly divided into four different scenarios: the scenario of qualitatively unsatisfactory specimens, the scenario of cytologic atypia (mostly nuclear atypia), the scenario of architectural atypia (microfollicular architecture) and the scenario of combined cytologic and architectural atypia (2,10,11).
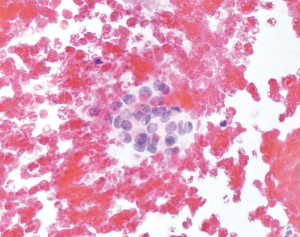
- The first scenario comprises all those specimens that suffer from preparation, fixation, and staining artifacts and that do not allow a complete evaluation of cellular details. Cells appear with nuclear enlargement that raise the suspicion for atypia, but a definitive diagnosis cannot be established. Also, specimens with poor cellularity and somehow atypical cells are placed into these scenarios. Atypia do not suggest the presence of any specific tumor type in particular (like in the two other scenarios) and can be epithelial, mesenchymal or lymphoid in origin or be non-specific. The cytopathological diagnosis would be AUS/FLUS in these cases (Figure 1);
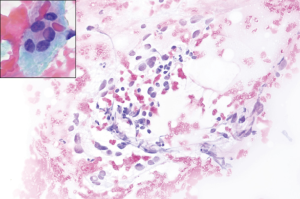
- The second scenario comprises specimens with cytological atypia, irrespective of the architecture of the cells. Usually specimens present with few cells with enlarged nuclei, chromatin pallor, sometimes grooves or vaguely nuclear vacuolization (mimicking nuclear pseudoinclusions). Atypical cells are so few that exclusion of malignancy as well as confirmation of malignancy [in the presence of papillary thyroid cancer (PTC)] is not possible. The presence of isolated oncocytes, without a clinical context that justifies their presence (i.e., Hashimoto’s thyroiditis), is also regarded as atypical. The cytopathological diagnosis would be AUS/FLUS in these cases (Figure 2);
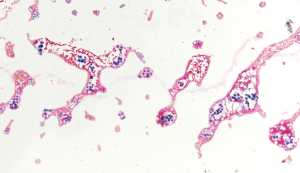
- The third scenario comprises specimens that contain microfollicular structures without nuclear atypia. If the specimen is poorly cellular and shows some microfollicles, a follicular neoplasm cannot be completely excluded. Moreover, some cases are clearly benign clinically, but have a microfollicular component that cannot be ignored. The cytopathological diagnosis would be AUS/FLUS in these cases (Figure 3). Conversely, if microfollicles are abundant and do not leave doubt concerning the presence of a follicular lesion, the cytopathological diagnosis would be FN/SFN in these cases. Also, specimens with abundant oncocytes, without any colloid or lymphocytic infiltration in the background, will be interpreted as FN/SFN, Hurthle cell type;
- The fourth scenario comprises cases with scant cellularity and presence of both nuclear atypia and microfollicular structures. The cytopathological diagnosis would be AUS/FLUS in these cases; most cases of NIFTP will be diagnosed within this scenario (12).
Cytology is considered a screening test in the two Bethesda DC of AUS/FLUS and FN/SFN, as it cannot be conclusive concerning a benign or malignant diagnosis. Conversely, cytology in the benign and the malignant DC is considered a diagnostic test, because negative predictive value (NPV) and positive predictive value (PPV) are high and close to 100%, respectively.
The NIFTP entity and its impact on the indeterminate DC
The NIFTP term was introduced in 2016 after an international multidisciplinary group of endocrine experts conducted a retrospective study confirming what had already been known for years; the encapsulated forms of follicular variant (FV)-PTC have indolent behavior with almost no adverse outcomes (5). Therefore, they proposed to treat such neoplasms with a simple lobectomy and no adjunct radioiodine radiation and/or completion thyroidectomy. The NIFTP concept was accepted and included into the latest version of the WHO classification of tumors of endocrine organs and endorsed by the American Thyroid Association (ATA) (1,13). The revised version of TBSRTC also took into account its presence, but did not create a specific DC for it, as NIFTP diagnosis is not possible on FNA (14). It is possible to suggest its presence, and to differentiate it partly from follicular adenoma (FA) and classical PTC. However, a definitive diagnosis can only be achieved by complete histological examination and inclusion in totality of the tumor capsule, in order to exclude capsular and vascular invasion and by complete histological examination of the lesion content, in order to exclude the presence of papillary structures (15,16). After the introduction of the NIFTP lesion and in order to avoid false positive diagnoses, i.e., to diagnose tumors lesions as malignant that are ultimately considered having a low malignant potential, the revised version of TBSRTC recommended to use strict criteria to diagnose PTC on FNAC, i.e., only in cases with papillary structures, abundant nuclear inclusions and psammoma bodies (2,14). The disadvantage of this recommendation is that, unfortunately, the indeterminate DC will increase in percentage compared to the other DC, as we want to avoid a false PTC diagnosis. It has been shown by several retrospective studies and in a recent meta-analysis, that the great majority of NIFTP cases actually fall into the AUS/FLUS DC, followed by the FN/SFN DC (12,17-19). In our opinion, the suggestion to limit the number of AUS/FLUS cases to 10% will be outdated.
Sonographic aspects
It is well known that thyroid ultrasound (US) cannot be confidently used to predict benignity nor malignancy of thyroid nodules, thus excluding its sole use in the management of patients with thyroid lesions. However, thyroid US is a valid tool in the malignant risk stratification of indeterminate thyroid nodules along with clinical features of patients. Some US features such as microcalcifications, hypoechogenicity, irregular margins or absent halo signs, a solid aspect, intranodular vascularization, and shape (taller than wide), when present together, can predict malignancy with a reasonable amount of certainty (13,20). Thus, the ATA sonographic patterns should be used not only to set the size-threshold for the biopsy but also to personalize the management after the biopsy (13,21). To implement the PPV of thyroid US, US examination should be extended to the central and latero-cervical lymph node compartment when thyroid nodules are evaluated, as suspected lymph nodes may push the global evaluation of a single nodule towards malignancy.
In a recent study of 342 patients diagnosed with AUS/FLUS during a 4-year period and with histological examinations available, the authors aimed to find some US features that could help predict malignancy in this indeterminate DC. To reflect how badly this DC was perceived, the authors of the study defined it as the “frustrating” DC (22). Actually, the ATA guidelines have suggested that US assessment of suspicious features can be a valid tool in the management of patients in the indeterminate DC (13). The authors found that hypervascularity was the most common suspicious US finding in patients with malignancy at histology (22). On the contrary, border irregularity was the most common US finding in patients with benignity at histology. Many other studies have on the contrary showed that other US characteristics were able to predict malignancy in the AUS/FLUS DC: microcalcifications, “taller than wide” diameter on transverse view, border irregularity, absence of halo and hypoechogenicity (23-25). Another technique, with great expectations in the past to be more effective in the evaluation of thyroid nodules, was elastography. This technique is based on the deformation characteristic of soft tissue when compressed by an external force. This distortion is achieved with an US beam and can be registered (26). Unfortunately, its use was not as successful as first thought.
Management
In the first version of TBSRTC, cases diagnosed as AUS/FLUS are supposed to be managed with repeat FNAC, while cases diagnosed as FN/SFN are supposed to be managed with diagnostic lobectomy (27). When dealing with poorly cellulated, poorly fixed and poorly stained smears, it is better to repeat the FNAC to obtain more cellular specimens and thus clarify the situation. Repetition of the FNAC in AUS/FLUS cases could establish a final diagnosis of benignity in greater than 50% of nodules (28,29). In the new version of TBSRTC, the possibility of a diagnostic lobectomy was introduced also for cases diagnosed as AUS/FLUS (2). In a study conducted prior to the new version of TBSRTC, it was shown that the ROM in cases with a diagnosis of AUS/FLUS sent directly to surgery was 15.7% and the decision for surgery appeared to be influenced by gender and age, not US characteristics (30). A recent article from The Brigham & Women’s Hospital showed that some patient-specific factors (i.e., contralateral nodules, hypothyroidism, fluorodeoxyglucose avidity on positron emission tomography scan, family history of thyroid cancer, and increased surgical risk) were associated with a preference by surgeons and patients for initial total thyroidectomy in case of cytological indeterminate nodules (31).
Factors that help further in stratifying the ROM of patients with indeterminate thyroid nodules and that can positively influence the decision to perform surgery, comprise a nodule size >4 cm, male sex, age of patients at the extremes, and prior head and neck irradiation (32). A meta-analysis performed on 3,494 patients with indeterminate thyroid nodules at FNAC calculated the odds ratio (OR) for gender and lesion diameter as a risk factor of malignancy. The authors found that the pooled OR for the male gender was 1.51, whereas the pooled OR for the female gender was 0.68; concerning nodule size, the pooled OR for nodule size >4 cm was 2.10, whereas the pooled OR for a size <4 cm was 0.48 (33). Conversely, a negative 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG-PET/CT) correctly predicted benignity on histopathology and can thus help avoid surgery due to its high NPV (34). An additional management option suggested in the revised version of TBSRTC is the possibility to perform molecular tests for patients diagnosed either with the AUS/FLUS DC or with the FN/SFN DC (2).
Molecular alterations
Discovery of any molecular alterations associated with thyroid cancer and the consequent implementation of molecular tests with such new markers, serve to refine the ROM associated with the indeterminate category with the benefit of patient management. Two different approaches characterize thyroid molecular tests. One aims to exclude (rule-out) and the other aims to confirm (rule-in) malignancy in the indeterminate category. It is important in both cases to know the prevalence of malignancy in AUS/FLUS and FN/SFN thyroid nodules at the respective institutions before the interpretation of the results, as prevalence affects sensitivity and the NPV of the test. The rule out test is offered by the Veracyte company and consists in the evaluation of several mRNA to optimize NPV and is called Afirma gene expression classifier (GEC). The rule-in test is developed by the University of Pittsburg and commercialized by CBLPath and known as ThyroSeq. It is based on next generation sequencing (NGS) for point mutations and gene fusions in known thyroid cancer related genes. Another test available and belonging to the rule-in tests is the ThyGenX-ThyraMIR commercialized by Interpace Diagnostics. It is also based on NGS for point mutations and gene fusions (ThyGenX) plus a panel of microRNA markers (ThyraMIR). Recently, new versions of the Afirma [genomic sequencing classifiers (GSC)] and ThyroSeq (ThyroSeq V3 Genomic Classifier) tests became available and both tests are now associated with higher diagnostic accuracy. The new and improved version of the Afirma GSC gives values of sensitivity of 91.1% and specificity of 68.3%, with an improvement of 36% for specificity in comparison to the previous version and with a cancer prevalence of 24% (35). The ThyroSeq V3 Genomic Classifier, showed high specificity and PPV allowing for diagnostic surgery to be avoided in more than 60% of patients with indeterminate results on FNAC. The prevalence of malignancy in this cohort was 30% and also comprises cases diagnosed as SM. In Bethesda nodules diagnosed as AUS/FLUS and FN/SFN, ThyroSeq V3 had a sensitivity of 94%, specificity of 82%, an NPV of 97%, and PPV of 66% with a prevalence of malignancy of 28% (36).
A different possibility to the application of commercially available molecular tests is to choose a test in function of the cytopathological features. This “personalized” approach saves money and takes into account the fact that usually cases placed into the AUS/FLUS DC are compromised either in terms of quantity or quality. If the predominant cytological features are atypical nuclei, then we would prefer to look for BRAF, H-N-K-RAS, RET/PTC alterations. On the contrary, if the predominant cytological features are the presence of non-atypical microfollicular structures, then we would prefer to look for H-N-K-RAS and PAX8/PPARg alterations (11). A recent meta-analysis found a BRAF mutation rate of 4–6% in indeterminate cytology (37). Thus, our “personalized”, cytopathologically driven approach could make BRAF genetic analysis more cost-effective in nodules with indeterminate cytology.
From a practical point of view, all kind of cytological specimens, preparations [including liquid-based cytology (LBC)], and all kinds of staining, can be successfully used to carry out molecular tests in the thyroid. FNAC provide good quality DNA. Even RNA based tests are feasible on stained material that can be analyzed for the detection of point mutations, chromosomal rearrangements (also with FISH), gene expression profiling, and miRNA profiling (38).
The application of molecular tests should not be conceived as a stand-alone test, but rather it should be considered as a relevant information to be integrated with the clinical and imaging data as an adjunct to patient’s management. If the indication for surgery is made a priori and independently of the molecular test results, for example because of the size of the nodule, it is not useful or cost effective to proceed with molecular tests (39).
Conclusions
In conclusion, the indeterminate cytological diagnosis, represented by cases in the AUS/FLUS and FN/SFN DC, comprises cases that do not fulfill the cytological criteria to be placed in a malignant category. The ROM is about 6–18% and 10–40% if NIFTP is not considered a carcinoma and 10–30% and 25–40% if NIFTP is considered a carcinoma, respectively. Evaluation of the patient’s history, ultrasonographic features, and results of molecular tests can help refine the ROM in this group of patients and better tailor management for each of them.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lloyd RV, Osamura RY, Kloppel G, et al. Tumours of the thyroid gland. In: Lloyd RV, Osamura RY, Kloppel G, et al. editors. World health organization classification of tumours of endocrine organs. Lyon: IARC, 2017:65-142.
- Ali SZ, Cibas ES. Editors. The Bethesda System for Reporting Thyroid Cytopathology. Definitions, Criteria, and Explanatory Notes. 2nd edition. New York: Wiley, 2017.
- Cross P, Chandra A, Giles T, et al. Guidance on the reporting of thyroid cytology specimens. 2nd edition. London, United Kingdom: Royal College of Pathologists, 2016.
- Bongiovanni M, Spitale A, Faquin WC, et al. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol 2012;56:333-9. [Crossref] [PubMed]
- Nikiforov YE, Ghossein RA, Kakudo K, et al. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features. In: Lloyd RV, Osamura RY, Kloppel G, et al. editors. World Health Organization Classification of Tumours of Endocrine Organs. Lyon: IARC Press, 2017:78-80.
- Parente DN, Kluijfhout WP, Bongers PJ, et al. Clinical Safety of Renaming Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: Is NIFTP Truly Benign? World J Surg 2018;42:321-6. [Crossref] [PubMed]
- Bychkov A, Keelawat S, Agarwal S, et al. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multi-institutional study in five Asian countries. Pathology 2018;50:411-7. [Crossref] [PubMed]
- Vaccarella S, Franceschi S, Bray F, et al. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med 2016;375:614-7. [Crossref] [PubMed]
- Lim H, Devesa SS, Sosa JA, et al. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017;317:1338-48. [Crossref] [PubMed]
- Bongiovanni M, Krane JF, Cibas ES, et al. The atypical thyroid fine-needle aspiration: past, present, and future. Cancer Cytopathol 2012;120:73-86. [Crossref] [PubMed]
- Bellevicine C, Sgariglia R, Migliatico I, et al. Different qualifiers of AUS/FLUS thyroid FNA have distinct BRAF, RAS, RET/PTC, and PAX8/PPARg alterations. Cancer Cytopathol 2018;126:317-25. [Crossref] [PubMed]
- Bongiovanni M, Giovanella L, Romanelli F, et al. Cytological diagnoses associated with non-invasive follicular thyroid neoplasms with papillary-like nuclear features, (NIFTP) according to the Bethesda System for Reporting Thyroid Cytopathology: a systematic review and meta-analysis. Thyroid 2018. [Epub ahead of print].
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Pusztaszeri M, Rossi ED, Auger M, et al. The Bethesda System for Reporting Thyroid Cytopathology: Proposed Modifications and Updates for the Second Edition from an International Panel. Acta Cytol 2016;60:399-405.
- Hung YP, Barletta JA. A user's guide to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Histopathology 2018;72:53-69. [Crossref] [PubMed]
- Nikiforov YE, Baloch ZW, Hodak SP, et al. Change in Diagnostic Criteria for Noninvasive Follicular Thyroid Neoplasm With Papillarylike Nuclear Features. JAMA Oncol 2018;4:1125-6. [Crossref] [PubMed]
- Kim M, Kim JE, Kim HJ, et al. Cytologic Diagnosis of Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features and Its Impact on the Risk of Malignancy in the Bethesda System for Reporting Thyroid Cytopathology: An Institutional Experience. J Pathol Transl Med 2018;52:171-8. [Crossref] [PubMed]
- Strickland KC, Howitt BE, Marqusee E, et al. The Impact of Noninvasive Follicular Variant of Papillary Thyroid Carcinoma on Rates of Malignancy for Fine-Needle Aspiration Diagnostic Categories. Thyroid 2015;25:987-92. [Crossref] [PubMed]
- Faquin WC, Wong LQ, Afrogheh AH, et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol 2016;124:181-7. [Crossref] [PubMed]
- Rago T, Vitti P. Role of thyroid ultrasound in the diagnostic evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab 2008;22:913-28. [Crossref] [PubMed]
- Valderrabano P, McGettigan MJ, Lam C, et al. Thyroid nodules with indeterminate cytology: Utility of the American Thyroid Association sonographic patterns for cancer risk stratification. Thyroid 2018;28:1004-12. [Crossref] [PubMed]
- Brown C, Mangano W, Thompson S, et al. Factors Predicting Thyroid Malignancy in Fine Needle Aspiration Biopsy Specimens Classified as Atypia of Uncertain Significance/Follicular Lesion of Uncertain Significance. Am Surg 2018;84:1207-13. [PubMed]
- Méndez W, Rodgers SE, Lew JI, et al. Role of surgeon-performed ultrasound in predicting malignancy in patients with indeterminate thyroid nodules. Ann Surg Oncol 2008;15:2487-92. [Crossref] [PubMed]
- Khoncarly SM, Tamarkin SW, McHenry CR. Can ultrasound be used to predict malignancy in patients with a thyroid nodule and an indeterminate fine-needle aspiration biopsy? Surgery 2014;156:967-70. [Crossref] [PubMed]
- Yoon JH, Kwon HJ, Kim EK, et al. Subcategorization of atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS): a study applying Thyroid Imaging Reporting and Data System (TIRADS). Clin Endocrinol (Oxf) 2016;85:275-82. [Crossref] [PubMed]
- Ophir J, Alam SK, Garra B, et al. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H 1999;213:203-33. [Crossref] [PubMed]
- Ali SZ, Cibas ES. editors. The Bethesda system for reporting thyroid cytopathology. Definitions, criteria and explanatory notes. New York: Springer, 2010.
- Faquin WC. Reply to can a gene-expression classifier with high negative predictive value solve the indeterminate thyroid fine-needle aspiration dilemma? Cancer Cytopathol 2013;121:404. [Crossref] [PubMed]
- Faquin WC. Can a gene-expression classifier with high negative predictive value solve the indeterminate thyroid fine-needle aspiration dilemma? Cancer Cytopathol 2013;121:116-9. [Crossref] [PubMed]
- Nagarkatti SS, Faquin WC, Lubitz CC, et al. Management of thyroid nodules with atypical cytology on fine-needle aspiration biopsy. Ann Surg Oncol 2013;20:60-5. [Crossref] [PubMed]
- Angell TE, Vyas CM, Barletta JA, et al. Reasons Associated with Total Thyroidectomy as Initial Surgical Management of an Indeterminate Thyroid Nodule. Ann Surg Oncol 2018;25:1410-7. [Crossref] [PubMed]
- Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am 2010;43:229-38. vii. [Crossref] [PubMed]
- Trimboli P, Treglia G, Guidobaldi L, et al. Clinical characteristics as predictors of malignancy in patients with indeterminate thyroid cytology: a meta-analysis. Endocrine 2014;46:52-9. [Crossref] [PubMed]
- Piccardo A, Puntoni M, Treglia G, et al. Thyroid nodules with indeterminate cytology: prospective comparison between 18F-FDG-PET/CT, multiparametric neck ultrasonography, 99mTc-MIBI scintigraphy and histology. Eur J Endocrinol 2016;174:693-703. [Crossref] [PubMed]
- Patel KN, Angell TE, Babiarz J, et al. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg 2018;153:817-24. [Crossref] [PubMed]
- Nikiforova MN, Mercurio S, Wald AI, et al. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018;124:1682-90. [Crossref] [PubMed]
- Trimboli P, Treglia G, Condorelli E, et al. BRAF-mutated carcinomas among thyroid nodules with prior indeterminate FNA report: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2016;84:315-20. [Crossref] [PubMed]
- Bode-Lesniewska B, Cochand-Priollet B, Straccia P, et al. Management of thyroid cytological material, preanalytical procedures and bio-banking. Cytopathology 2019;30:7-16. [Crossref] [PubMed]
- Paschke R, Cantara S, Crescenzi A, et al. European Thyroid Association Guidelines regarding Thyroid Nodule Molecular Fine-Needle Aspiration Cytology Diagnostics. Eur Thyroid J 2017;6:115-29. [Crossref] [PubMed]


