
Implant selection in the setting of prepectoral breast reconstruction
Introduction
The goal of breast reconstruction is to recreate a breast that looks and feels like the natural breast. As in the majority of cases the breast is reconstructed using an implant-based approach (1), the implant and its characteristics to a large extent define the recreated breast size and form. Much research has been focused on improving implant designs, particularly silicone implants, over the last decade. As a result, there is a wide variety of implants. Although it is good to have choices, it makes selecting an implant that best suits the patient a challenging task.
Implant selection can be viewed as both a science and an art. It can be performed objectively by tissue-based measurements but what constitutes an ideal breast size and form is a subjective perception influenced by personal preference and cultural norms. In reality, the implant selected is often a compromise between tissue-based measurements and patient preference.
In this article, the authors review the science behind implant selection, including tissue-based measurements as well as implant types as an understanding of implant characteristics is equally critical to selecting the appropriate implant.
Overview of breast implants
Saline implants
Saline implants were initially developed for introduction via a small incision while deflated (2). As the implant is inflated after insertion, it provides the flexibility of adjusting the fill volume. With a slight overfill a greater breast projection and upper pole fullness can be achieved. Aggressive overfilling, however, should be avoided as it may result in a more spherical shape and scalloping along the implant edge, with knuckle-like palpability and unnatural firmness. Compared with silicone implants, saline implants allow easy detection of ruptures as they deflate and are less expensive. The disadvantage of saline implants is their less natural feel as the consistency on palpation resembles that of water rather than the viscous feel of natural breast tissue. They have a higher risk of rippling and rupturing than silicone implants and can cause bottoming out over time due to the distribution of the weight of the implant at the lower pole.
Silicone implants
All currently available silicone implants are fourth or fifth generation implants, designed under more stringent American Society for Testing Methodology (3) and Food and Drug Administration guidance on shell thickness and gel viscosity and manufactured under improved quality control measures (4) compared with earlier generation implants. A wide selection of silicone implants is available from three implant manufacturers [MENTOR, Allergan (Madison, NJ, USA), and Sientra (Santa Barbara, CA, USA)] that vary in shell surface, cohesivity (or viscosity) of silicone filler, shape, size (volume), and projection (profile) (5-7).
Silicone implants have a smooth or textured surface. The textured surface was created with the aim of stabilizing the implant within the pocket. The pores making up the textured surface allow tissue adherence creating an adhesive effect which holds the implant in place. Implant stabilization appears to correlate with pore size with larger pores having a greater adhesive effect than smaller pores (8). The three implant manufacturers have their own proprietary textured surface.
All modern day silicone implants are cohesive implants; that is the silicone filler is not liquid silicone but viscous silicone. The viscosity or cohesivity of the silicone filler is determined by the degree of crosslinking of the silicone polymer chains; the greater the degree of crosslinking, the greater the cohesivity. The highly cohesive implants are able to maintain their shape and dimension, that is, the gel distribution within the implant does not change when held vertically or horizontally (Figure 1). These implants are often referred to as “form-stable” implants, although no implant is truly form-stable. The form-stable implants are generally fifth generation implants while the less cohesive implants are generally fourth generation implants.
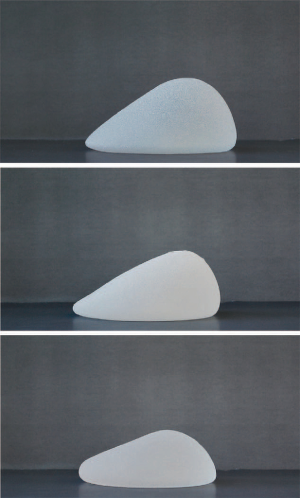
Silicone implants are available in round or anatomic (“tear drop”) shapes. In round-shaped implants, in a horizontal position, the gel is distributed evenly within the implant. In an upright position, the gel descends to the lower pole, resulting in a centrally full appearance to the reconstructed breast and a collapsed upper pole. However, the extent to which the gel descends is dependent on the cohesivity of the gel; the greater the cohesivity, the more even the distribution of the gel in an upright position and the fuller the upper pole (Figure 2). It is also directly related to the type of soft tissue support that is present at the lower pole; the better the support, less gel will migrate to the lower pole. In addition, the tighter the pocket the more influence the implant will have on shaping the pocket.
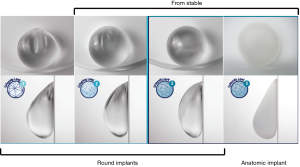
In anatomically-shaped implants, there is a greater concentration of gel in the lower half of the implant, leading to fullness in the lower pole and a gentle slope in the upper pole that resembles the natural breast shape (Figure 2). In addition to the cohesivity of the gel, the gel-shell fill ratio is another factor that contributes to maintenance of implant shape. The more cohesive the gel, the higher the gel-shell fill ratio and the more likely the implant will maintain its shape.
All anatomically-shaped implants are highly cohesive form-stable implants. These implants have a textured surface to reduce the risk of implant rotation. Because of their thicker viscosity, the form-stable implants are stiffer than the less cohesive implants and hence the risk of rippling is lower with these implants. Round-shaped implants, on the other hand, are available in both textured and smooth surfaces and have different degrees of gel cohesivity, ranging from cohesive to highly cohesive. Implants with a lower cohesivity are softer than implants with higher cohesivity but are associated with a higher risk of rippling. The new generation of round-shaped implants (Allergan Natrelle INSPIRA® and Sientra High-Strength Cohesive Plus) that have higher cohesive gel fills are form-stable implants (Figure 2). These implants retain the upper pole fullness much better than the less cohesive implants based on the lower pole support that is present.
Each type of silicone implant has a range of profiles—typically low, moderate, and full profile. It is important to note that within each profile type, the gel-shell fill ratio varies across the implants from the different manufacturers. For example, an implant with moderate projection from Mentor does not have the same gel-shell fill ratio as an implant from Sientra.
Principles of implant selection
Given the wide variety of implants available in the market, the selection of an appropriate implant can be overwhelming for both the patient and the surgeon. There are, however, some basic principles that underlie implant selection, which apply to both saline and silicone implants. Firstly, implant selection should be individualized as the same implant in two different individuals may look very different. Secondly, implant selection should be guided by tissue-based measurements, extent of soft tissue coverage, and patient desire.
Tissue-based measurement
Tissue-based measurements or dimensional planning is an objective, analytic approach to implant selection that is based on the individual patient’s anatomy and proportions. The patient’s chest and breast measurements, waist and shoulder proportions, and posture are the relevant anatomical considerations for dimensional planning. Chest and shoulder proportions as well as posture are important considerations when selecting the implant height and projection. For example, a low-height implant would be more suitable for a patient with a wide chest and shoulders; while a patient with a narrow chest and shoulders would benefit from a full-height implant. For a patient with concave posture, a high-projection implant would be more suitable and for a patient with convex posture, a low-projection implant would be a better choice. Chest wall deformities and/or asymmetry can also impact implant selection and should be assessed in all patients.
Breast measurements of relevance in implant selection include breast base width, nipple to inframammary fold (IMF) distance, intermammary distance, and sternal notch to nipple distance (Figure 3). The size, height, and projection of the implant are guided by these breast measurements. The breast width, measured from the anterior axial line to the beginning of the intermammary distance, is instrumental in determining the implant base width and, hence, the size of the implant. The intermammary distance impacts the volume of the implant. The nipple to IMF distance, measured under maximum stretch, guides in determining the height and projection of the implant. The sternal notch to nipple distance impacts the height of the implant and how the chest fits the overall anterior trunk.
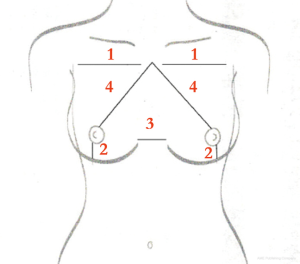
In addition to breast measurements, the use of external sizers and three-dimensional imaging can further help guide objective implant selection. Imaging allows for precise tissue-based implant selection (9) but has not yet been widely adopted due to the cost associated with it.
Soft tissue coverage
Soft tissue thickness after mastectomy and the degree of skin laxity are also important considerations in implant selection. Soft tissue thickness is determined by the soft tissue pinch test which is performed in the medial upper pole of the breast. Soft tissue thickness helps guide the decision to select a less or more cohesive implant. A less cohesive implant is usually more suitable in a patient with thick soft tissue coverage while a more cohesive implant is more suitable in a patient with thin soft tissue coverage regardless of the shape of the implant (round or anatomic). In some cases, soft tissue thickness can be augmented with fat grafting that can improve the overall aesthetic shape of the breast.
It is of importance to note that soft tissue thickness should not be confused with skin quality and viability. Patients with poor skin quality or viability (i.e., poor perfusion) are not candidates for immediate prepectoral (or dual-plane) reconstruction (10) and they are better served with a delayed reconstructive approach.
Skin laxity (or skin stretch) is measured by grasping the skin of the medial areola and pulling the breast maximally anteriorly. The anterior-posterior excursion determines the skin stretch distance. The skin stretch distance plays an important role in the selection of a suitable implant size. In general, skin laxity allows for the use of a larger implant but implant visibility or palpability is a concern as is the risk of lower pole descent over time. This is also important when planning expansions and over-expansions should be eliminated in non-radiated reconstructed breasts.
Patient desire
Implant selection should be performed in consultation with the patient and at the preoperative planning stage. Patient desires regarding reconstructed breast size, shape, projection, and height should be assessed and discussed with the surgeon’s dimensional measurements. Ideally, a compromise between the patient’s desire and the patient’s anatomical dimensions should be reached. It is important to note that although there may be an optimal implant type for a particular patient based on dimensional or tissue-based planning, the patient may not desire that implant type and may opt for a larger or smaller implant with more or less projection. The surgeon’s role is to counsel the patient on the optimal option but ultimately the patient’s desire needs to be respected.
Authors’ guide to implant selection
The authors typically use round-shaped or anatomically-shaped silicone implants in all their patients undergoing prepectoral reconstruction. Saline implants are generally not recommended for these cases. Implant rippling and wrinkling that are commonly associated with saline implants, due do to their liquid fill, are more obvious when implants are placed subcutaneously and are further accentuated with thin mastectomy flaps. Saline implants are only used if specifically requested by patients and when they have sufficient soft tissue coverage or sufficient fat depots for autologous fat grafting to conceal rippling or wrinkling. Alternatively, the subpectoral approach is offered to these patients.
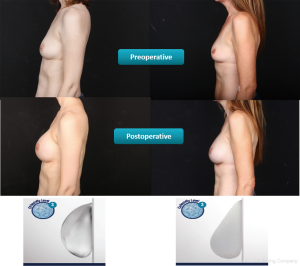
The thickness of soft tissue coverage is used to guide the choice between less or more cohesive implants (Figure 4). Generally, round less cohesive implants are better suited for patients with thick soft tissue coverage. As these patients usually have a high body mass index (BMI), they would benefit from greater breast projection which can be achieved with a round less cohesive implant. When held in an upright position, a round less cohesive implant will have more of a collapse (due to less cohesivity) at the upper pole due to descend of the gel from the upper to the lower pole (Figure 5). The greater volume of gel at the lower pole makes the implant project forward. The loss of gel at the upper pole is not an issue as these patients have thick subcutaneous tissue. In addition, these patients usually have sufficient fat depots for autologous fat grafting, if needed.
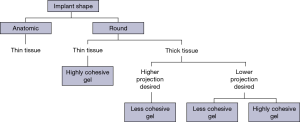
Generally, more cohesive implants are better suited for patients with thin soft tissue coverage. These patients usually have a low BMI and would benefit from the form stability that provides some fullness in the upper pole and the patient can choose between a round shape or anatomic shape (Figure 6, right). The highly-cohesive round implants do not collapse in an upright position (Figure 5, right) and provide upper pole fullness (Figure 6, left).
The authors typically perform prepectoral reconstruction as a two-staged procedure because the expander stage provides an opportunity to create the footprint of the breast for implant placement at the second stage. The acellular dermis used in the first stage for expander coverage serves to stabilize the pocket and the footprint of the breast. In fact, the incorporated acellular dermis may be envisaged as the capsule that defines the pocket dimension and this capsule is preserved and not released at the second stage. The expander also serves as a temporary sizer that allows the patient flexibility in shaping their breast at the second stage by selecting from the various degrees of gel cohesivity and projections that are available for the selected implant base width. Thus, decisions regarding projection and gel cohesivity do not have to be made early when the patient is overwhelmed with her cancer diagnosis. By using the expander as a “temporary sizer”, the patient can take her time and make decisions postmastectomy by utilizing the existing expander as a “communication tool” to convey her desires.
Typically, for a planned round implant, a full-height expander and for a planned anatomic implant, a short-to-moderate height expander is used at the first stage. When reconstruction in a prepectoral plane is planned then the goal is to set the dimension of the new pocket (based on base width) and the new footprint with the dermal matrix and expander construct. Once the dermal matrix is incorporated, the goal is not to interrupt this newly formed soft tissue support for the future implant. This will minimize any possible malposition that can occur following releasing the pocket to gain height or width. Therefore, selecting a tall expander can easily accommodate the round implant since the height of this implant cannot be controlled as it is a two-dimensional implant. For example, when an expander with a given base width of 13.0 cm is chosen then the appropriate planned round implant will have a base width of 13.25–14.0 cm. Since the height of a full height expander for a given 13.0 cm base expander is 13.5 cm, with some manufactures, then no release of dermal matrix will be required if a wider round implant of the appropriate dimensions is chosen, as the height is already included in the pre-planning. When an anatomically shaped implant is planned then a shorter height expander is utilized depending on the height of the anatomical implant that is chosen. There is a great deal of thought process that is involved in the preoperative phase to choose the appropriate expander so that during the second stage a simple efficient exchange to the permanent implant is performed without release of the new footprint.
Expanders are typically selected with a base width that is 0.5–1.0 cm narrower than the base width of the final implant. They are filled to about 60–80% of their maximum capacity and rarely to full capacity with the exception of radiated cases where the expander is filled to capacity. Underfilling the expander and using a narrower expander base width ensure a tight fit of the selected implant within the pocket. A tight fit is not only important with anatomically-shaped implants but it is also important with round-shaped implants as it attenuates the risk of anterior-posterior malposition. The basic principles of creating a tight pocket that is narrower than the final implant and underfilling the expander should be followed regardless of the shape of implant that is chosen.
Conclusions
Implant selection is a complex process that is guided by objective tissue-based measurements as well as subjective patient preferences related to perceptions of breast aesthetics. Generally, breast measurements provide a basis for the selection of implant size, height, and projection while the decision to use round or anatomical implants is based on the patient’s soft tissue coverage. When using round implants, it is important to consider gel cohesivity as it can influence the extent of upper pole fullness and projection. The systematic use of objective measurements for implant selection provides a means to control outcomes but patient preferences should not be overlooked as patient satisfaction ultimately defines reconstructive success.
Acknowledgements
Writing and editorial assistance was provided by Kalanethee Paul-Pletzer, PhD.
Footnote
Conflicts of Interest: Allen Gabriel, MD and G. Patrick Maxwell, MD are consultants for Allergan, Madison, NJ, USA.
References
- American Society of Plastic Surgeons. 2017 plastic surgery statistics report. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed October 25 2018.
- Clinical trial and evaluation of a proposed new inflatable mammary prosthesis. Plast Reconstr Surg 1972;50:220-6. [Crossref] [PubMed]
- Physical characterization of unimplanted gel filled breast implants. Should old standards be revisited? ASAIO J 1994;40:943-58. [Crossref] [PubMed]
- Manufacturing of mammary implants: a manufacturing of high technology. Ann Chir Plast Esthet 2005;50:394-407. [Crossref] [PubMed]
- MENTOR. Mentor breast implants. Available online: , Accessed November 2018.https://www.breastimplantsbymentor.com/breast-implants/implant-types
- Allergan. Breast reconstruction with Natrelle® silicone-filled breast implants and Natrelle Inspira® breast implants. Available online: https://www.natrelle.com/Content/pdf/natrelle-and-inspira-recon-get%20the%20guide.pdf. Accessed November 2018.
- Sientra. OPUS™ breast implants. Available online: http://sientra.com/breast-implants. Accessed November 2018.
- Comparison of the capsular response to the Biocell RTV and Mentor 1600 Siltex breast implant surface texturing: a scanning electron microscopic study. Plast Reconstr Surg 2001;108:2047-52. [Crossref] [PubMed]
- Preoperative implant selection for two stage breast reconstruction with 3D imaging. Comput Biol Med 2014;44:136-43. [Crossref] [PubMed]
- Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg 2017;139:287-94. [Crossref] [PubMed]


