
The impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on the diagnosis of thyroid nodules
Introduction
In one of his most popular books entitled “Human Tumors” (1), the renowned French-Canadian pathologist Prof. Pierre Masson [1880–1959], recognized as a pioneer in his field and as “the father of histopathology teaching in Canada”, advised us that “No classification is more difficult to establish than that of thyroid carcinomas… Of all cancers, they teach, perhaps, the greatest lessons of humility to histopathologists.” In 2016, almost 70 years later, the reclassification of a group of low-risk tumors known as non-invasive encapsulated follicular variant of papillary thyroid carcinoma (FVPTC) into a new entity, non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) (2,3), demonstrates the veracity of this statement. As such, the renaming of a malignant entity as a nonmalignant (albeit not benign) neoplasm has contributed to optimizing patient care by deescalating treatment and follow-up for an indolent neoplasm, decreasing medical expense and complications possibly caused by further treatment including radioactive iodine, and reassuring patients with this diagnosis. At the same time, NIFTP has significant implications not only for the practice of thyroid cytopathology but also for surgical pathology and for molecular tests, creating significant new challenges (4,5). Within 2.5 years, there have been more than 150 publications with a keyword of NIFTP according to a PubMed literature search on November 1st, 2018 (https://www.ncbi.nlm.nih.gov/pubmed/?term=niftp). These publications cover many aspects on this new tumor entity, including cytological and histological diagnosis, ultrasound (US) features, molecular genotyping, clinical management and long-term outcome of NIFTP patients. In this review, we focus on the impact of NIFTP on the diagnosis of thyroid nodules, starting with a case study.
Case study
A 34-year-old female underwent a thyroid fine needle aspiration (FNA) biopsy for a 2.5-cm nodule in the right lobe. Cytological evaluation showed moderately cellular smears composed of atypical follicular cells arranged essentially in microfollicles. The cells had moderate to marked nuclear atypia including enlargement, nuclear membrane irregularities with several grooves, and chromatin pallor (Figure 1A). However, no nuclear pseudoinclusions, nor papillary structure or psammoma body were identified. The FNA was diagnosed as: “Malignant, papillary thyroid carcinoma (Bethesda diagnostic category VI)” with the following explanatory note: “a follicular variant of papillary thyroid carcinoma (FVPTC) is favored; with the recent reclassification of a subset of indolent FVPTC as non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), the positive predictive value of the malignant category for thyroid FNA is expected to drop from 99% to 94–96%.” Thus, a small proportion of cases diagnosed as malignant by FNA may prove to be NIFTP upon histologic examination.” Subsequently, the patient underwent a repeat FNA for molecular testing with ThyGenX®, which assesses the most common genetic alterations across eight genes associated with papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC), including TERT promoter mutation. PAX8/PPARƔ rearrangement was found in isolation, which was highly suggestive of a low-risk follicular neoplasm (FN), mainly FVPTC or NIFTP and less commonly FTC of follicular adenoma (6). A right hemithyroidectomy with central compartment lymph node dissection was performed, and the histological examination of the thyroid nodule showed an invasive encapsulated FVPTC (EFVPTC) with both vascular and capsular invasion (Figure 1B,C,D). The tumor was limited to the thyroid gland without lymph node metastasis (pT2 pN0). No adjuvant treatment was given to the patient, according to the American Thyroid Association (ATA) guidelines 2015 (7) for low-risk thyroid carcinomas.

What is NIFTP?
The incidence of thyroid cancer diagnoses has tripled over the past 30 years, and PTC has accounted entirely for this increase (8). In contrast, the mortality rate has remained very low, indicating the indolent clinical behavior of the vast majority of thyroid cancers which were identified during that time (8). The term overdiagnosis has been applied to this concept of finding subclinical disease that is not likely to progress. In fact, the increased incidence of PTC is related to increased detection of small tumors including microcarcinomas (<1 cm) and a shift in pathological diagnostic criteria over time with the increased recognition and diagnosis of FVPTC. Specifically, the prevalence of the EFVPTC has increased and this entity accounted for 10–25% of all newly diagnosed thyroid cancers in Europe & North America a few years ago (9,10). Prior to the introduction of the NIFTP nomenclature, all encapsulated follicular pattern tumors with nuclear features of PTC, even without invasive growth, were categorized as malignant tumors (i.e., non-invasive EFVPTC) (9,10). Since the nuclear features of PTC are subjective, the diagnostic reproducibility of EFVPTC, even among experts, was historically extremely poor (9-11). For about 30 years, it was wrongly assumed that EFVPTC behaved and spread like its classical counterpart and, therefore, that it should be treated likewise. As an example, in the first edition of the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) from 2009 (12), it was stated that “FVPTC behaves clinically in a manner indistinguishable from conventional PTC”. Therefore, a major challenge of cytopathologists at that time and before the era of NIFTP, was to diagnose this entity as malignant or at least suspicious for malignancy (SFM), in order to limit the rate of false negative diagnoses. During the last decade, however, it was found that FVPTC is a heterogeneous group of tumors and that the clinical behavior and molecular features of the non-invasive EFVPTC, the most common subtype of FVPTC, resemble those of the follicular adenoma/carcinoma group of tumors (RAS-like) rather than the classic PTC group of tumors (BRAF-like) (2,9-10). It was found that EFVPTC is indolent when there is no vascular or capsular invasion, akin to follicular adenoma. On the other hand, encapsulated invasive FVPTC (with capsular and/or vascular invasion) is essentially identical to FTC in its behavior, molecular profile and pathological criteria, except for the nuclear changes of PTC. Therefore, similar to EFVPTC and NIFTP, some authors have suggested to rename this entity as well (e.g., FTC with nuclear atypia?), if additional studies show that they are indeed identical to FTC (13). The NIFTP nomenclature was subsequently introduced in 2016 by an international multidisciplinary group of endocrine pathology experts, on the basis of a retrospective study of a large number of cases of non-invasive and infiltrative EFVPTCs (2). The consensus term NIFTP that they proposed refers to a non-invasive neoplasm of thyroid follicular cells with a follicular growth pattern and nuclear features of PTC. By removing the word “carcinoma”, the main goal of this new nomenclature is to optimize patient care by deescalating treatment and follow-up, decrease medical expense and complications possibly caused by further treatment including completion thyroidectomy and radioactive iodine, while at the same time reassuring patients with this diagnosis who would no longer have the psychological stigma of cancer and its collateral damages (2,3,5). Importantly, NIFTP is considered a neoplasm with extremely low malignant potential and is best considered a premalignant lesion (borderline), rather than a purely benign or a frankly malignant lesion. The diagnosis of NIFTP is based on a combination of very strict inclusion and exclusion histologic criteria (2,5,14-16), namely on the finding of an encapsulated or sharply demarcated nodule with complete lack of invasive characteristics and cells with a follicular growth pattern showing nuclear features of PTC, without any well-formed papillary structures (even if up to 1% of papillae was initially accepted), psammoma bodies, significant solid growth (>30%), high mitotic rate, or tumor necrosis. The presence of other growth patterns indicative of aggressive variants of PTC, such as tall cell, columnar cell, and hobnail variants, also excludes the diagnosis of NIFTP. Because a definitive diagnosis of NIFTP can be made only after exclusion of invasion and the other features mentioned above, a complete histologic examination of the tumor capsule and/or tumor interface is required. Some NIFTP have a thick capsule, while a subset has a thin or incomplete capsule, and another subset have no capsule entirely but are well circumscribed with a sharp interface with adjacent thyroid parenchyma. Unlike classical PTC and most other variants of PTC, the nuclear atypia is typically more subtle in NIFTP, and range from diffuse in distribution to patchy (so called “sprinkling sign”) and multifocal. Some of the exclusion criteria for NIFTP, such as no papillae, no psammoma bodies, and no tumor necrosis, also suggest that the entire tumor should be submitted along with the capsular area (5). In most recent updates, the diagnostic criteria have been slightly revised, which now require no well-formed papillae and no BRAFV600E mutation, RET/PTC rearrangement or high-risk mutations such as TERT promoter (see below) (14-16). The new term NIFTP has been recommended for use by many professional societies including ATA (17), and is included as a new chapter on borderline tumors (i.e., ICD-O behavior code 1: neither benign nor malignant) of follicular cell origin in the latest “WHO Classification of Tumours of Endocrine Organs” published in 2017 (18). Since NIFTP is a new entity, it is expected that the diagnostic criteria will be further refined when more data becomes available in the future.
How common is NIFTP?
A recent meta-analysis found that NIFTP is averaging 9.1% of all PTCs worldwide (19), which is lower that what was initially suggested (2). However, the prevalence of NIFTP varies significantly among different areas, countries and institutions around the world, ranging from <1% up to 28% of all thyroid cancer diagnosis in retrospective studies [thoroughly reviewed by Bychkov et al. (19)]. Especially, the incidence of NIFTP in the Asian population is much lower than that in the non-Asian series. Such difference could be attributed several factors including: (I) various histological diagnostic criteria and thresholds for follicular-patterned thyroid lesions, which diagnostic reproducibility even among experts has been shown to be relatively poor; (II) geographic factors (e.g., iodine content) and ethnic (genetic traits) background which may influence the type, molecular mechanisms and mutation profile of PTCs. For example, the high prevalence of BRAFV600E mutation in Korean and Japanese patients is associated with a predominance of classic PTC over FVPTC; (III) variations and biases in the retrospective studies, including different ways to identify and to count NIFTP (e.g., based on the number of all thyroid malignancies or only PTC cases, with or without incidental microcarcinomas in the total number of cancers); and (IV) different approaches in the management of cytologically indeterminate thyroid nodules between Western and Asian countries (see below).
How does NIFTP impact cytology?
The reclassification of the non-invasive EFVPTC (“malignant”) as NIFTP (“nonmalignant”) has significant implications, for the diagnosis of thyroid nodules at the cytological, histological, ultrasonographic and molecular level (4,5), especially in areas of the world where the prevalence of NIFTP is high such as North America, Brazil and some countries in Europe. In contrast, in Asia where the proportion of non-invasive EFVPTC is very low, ranging between 0.3–0.4% in China up to 4.7% in Taiwan, the impact of NIFTP for the diagnosis of thyroid nodules will be minimal (19,20).
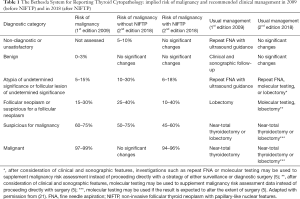
The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) (12,21) provides a standardized framework for the classification of thyroid FNA specimens based on cytomorphologic criteria, and has been endorsed by the latest 2015 ATA guidelines for the management of patients with thyroid nodules (7). Each of the 6 interpretive categories of TBSRTC is associated with an approximate risk of malignancy (ROM), which clinicians use to guide management of patients with a thyroid nodule (Table 1). The most significant impact of NIFTP reclassification is a decrease in the implied ROM, particularly for cases classified into 3 of the so-called “indeterminate” diagnostic categories of TBSRTC where the vast majority of NIFPT cases (90%) are clustered (22-26): (I) category III—atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS); (II) category IV—FN/suspicious for a FN (FN/SFN); and (III) category V—SFM (Table 1). Recent studies suggest that the decrease in ROM as a result of NIFTP will be most significant (up to 50%) for thyroid FNA specimens classified as SFM (22-26). In contrast, there is no significant change in the ROM for FNA specimens classified as benign, and only a small decrease (3–4% on average based upon a limited number of retrospective studies) for the malignant category. As an example, Faquin et al. (22) reported a series of 6,943 thyroid FNA specimens pooled from five institutions and found that 173 were diagnosed as NIFTP in the surgical resection specimens out of 756 malignant cases (23%). The preoperative FNA cytological diagnoses for these 173 NIFTPs were characterized as: nondiagnostic (1%), benign (9%), AUS/FLUS (31%), FN/SFN (27%), SFM (24%), and malignant (9%). When NIFTPs are considered nonmalignant lesions, the ROM is decreased by 1.4%, 3.5%, 13.6%, 15.1%, 23.4%, and 3.3%, in each Bethesda category (I to VI), respectively. Nonetheless, there are arguments against associating NIFTP’s new nonmalignant status with benignity and with revised ROMs. Although NIFTP is considered an indolent neoplasm with very low malignant potential, surgery (i.e., lobectomy) is considered the standard of care (5,17) and is necessary to establish the diagnosis of NIFTP definitively. Given this, it may not make sense to cluster NIFTPs with histologically benign nodules, for which nonsurgical follow-up is generally accepted in contrast to NIFTP (7,17). Acknowledging these conflicting perspectives, the second edition of TBSRTC (21) offers two updated versions of the ROMs: one that considers NIFTP as benign and another one that considers NIFTP as malignant (Table 1). ROMs that include NIFTP may be more meaningful clinically in countries where they are operated. In contrast, ROM without NIFTP may further support conservative management in some countries where borderline tumors are preferably followed-up clinically and radiologically (27) (see below). In the end, this binary (“black and white”) benign versus malignant histopathologic separation, although useful for statistical analysis and management purposes, may not be accurate in the sense that it does not reflect the progressive nature of thyroid neoplasia, which now includes a borderline category (“the gray zone”).
Full table
Can we diagnose NIFTP preoperatively?
It is currently not possible to diagnose NIFTP preoperatively by a single marker or a single technique (5). As exemplified in our case study, a combination of cytologic, ultrasonographic and molecular features can suggest the possibility of FVPTC or NIFTP or rule out this possibility in favor of classic PTC for example, but the definitive diagnosis requires complete histologic examination as discussed above. The key features of each individual preoperative test with regards to NIFTP diagnosis are discussed below.
Cytologic features of NIFTP
By definition, NIFTP is a hybrid neoplasm with significant cytomorphological overlap with FNs and classic PTC. FNAs of NIFTP are characterized by a microfollicular architecture along with variably nuclear features of PTC, including enlargement, crowding, contour irregularities with grooves, and chromatin clearing (5,28-34). In contrast, nuclear pseudoinclusions are rare (if any), and papillary structures and psammoma bodies should be absent (by definition). Additionally, and importantly, the nuclear features of NIFTP are generally more subtle than those of classical PTC, but more obvious than in benign follicular nodules (21,28-34). Because of sampling effects and tumor heterogeneity, they can be focal or widespread in the FNA specimen. For this reason, most NIFTP are diagnosed into one of the indeterminate Bethesda categories: AUS/FLUS, FN/SFN or SFM (22-26,28-34). As such, FNA can be considered as a good screening test for NIFTP since the majority of these cases will be triaged for surgery. Similar to FTC, the possibility of NIFTP may be raised preoperatively on cytology. However, the diagnostic confirmation of NIFTP can be made only on the surgical specimen. While cytopathologic ability to distinguish NIFTP from invasive FVPTC is not possible, cytological features can be used to help exclude a diagnosis of NIFTP in favor of a classical PTC diagnosis (28-34). In a review of 52 patients, Strickland et al. showed that the majority of NIFTPs and other follicular lesions such as follicular adenoma can be distinguished from classical PTC on cytopathology (29). NIFTP cases tended to exhibit a microfollicular growth pattern, whereas classical PTC cases tended to demonstrate papillae, pseudoinclusions, or psammomatous calcifications. Classical PTCs were accurately diagnosed preoperatively by cytopathology in 95% of cases. Brandler et al. found that NIFTP should be considered when PTC nuclear features and microfollicles are present (28). Bizzarro et al. found that NIFTP usually lacks pseudoinclusions and papillary structures (30). Howitt et al. also found statistically significant differences in cytomorphologic features between NIFTP and PTC (25); among PTC cases, 96% demonstrated tumor sheets, 50% had papillae, 79% had pseudoinclusions, and microfollicles were seen in only 4% of cases. In contrast, NIFTP demonstrated tumor sheets in 36% of cases, and microfollicles in 55% of cases, while papillae or pseudoinclusions were absent from all cases. Similarly, Renshaw and Gould. demonstrated that both papillae lined by cells with nuclear features of PTC and cellular swirls are highly specific for the diagnosis of PTC, since neither of them are seen in NIFTP (35). In a small subset of cases, however, cytopathologists may not be able to favor classical PTC or NIFTP/infiltrative FVPTC (IFVPTC). For these cases, US correlation and molecular testing may help to determine the best initial surgical approach (36,37).
Molecular features of NIFTP
Since molecular testing for indeterminate thyroid nodules is addressed in another chapter of this focused issue, we focus herein on the molecular features of NIFTP and molecular testing in the context of NIFTP.
The use of molecular testing on FNA material can assist in suggesting the diagnosis of NIFTP (5,36,38-43). Most cases of NIFTP demonstrate molecular clonal alterations which support the concept that NIFTP is a true neoplastic entity despite its indolent behavior (Table 2) (2). NIFTPs are primarily associated with activating mutations of 1 of the 3 RAS genes (NRAS > HRAS >> KRAS), with a frequency of 36–67%. Other driver mutations/alterations identified in NIFTP include PAX8-PARG rearrangement (4–22%), THADA (thyroid adenoma associated) fusions (22%), EIF1AX (7%, often coexisting with RAS mutation), and occasionally BRAFK601E mutations (4%) (2). However, none of the mutations present in NIFTPs are pathognomonic of this entity (43). Although RAS mutations are the dominant alteration in NIFTPs, they are also common in follicular adenomas and carcinomas, FVPTC (encapsulated and invasive) (Table 2), as well as poorly differentiated and anaplastic thyroid cancers. Similarly, PAX8-PPARG is also found in FTC and invasive FVPTC (Table 2) (6), as exemplified in our case study. The fact that NIFTP, non-invasive EFVPTC, and invasive EFVPTC show similar molecular profiles suggests a common pathway of progression in encapsulated follicular-patterned tumors, with or without nuclear features of PTC. Thus, the presence of any of these mutation in a preoperative cytological specimen does not allow for the definitive diagnosis of NIFTP. In contrast, a distinctive aspect of NIFTP lesions is the absence of BRAFV600E mutation, TERT promoter mutation, RET/PTC rearrangement and other mutations/fusions associated with classical and tall cell variants of PTC (2,5,38-43). These mutations should not be found in appropriately diagnosed NIFTPs, and therefore, their presence in a preoperative cytological specimen essentially rules out NIFTP. Molecular testing has been endorsed by the 2015 ATA guidelines as an alternative option in managing thyroid nodules with an indeterminate diagnosis on FNA (7), in particular the AUS and the FN/SFN categories, but it may also be considered for SFM and Malignant if the results are expected to changes the management (i.e., extent of surgery). A number of molecular tests have been studied and validated, essentially before the era of NIFTP, and are available for clinical usage. The most popular ones in North America are the proprietary Thyroseq®, a targeted Next-Generation Sequencing, and Afirma®, a Gene Expression Classifier (42). Unfortunately, these expensive tests are currently not available in many other countries. All the common mutations and fusions detected in NIFTP can be captured by Thyroseq®. The introduction of NIFTP as a non-malignant tumor changes the performance of molecular tests (44). Therefore, the utility, predictive value, and reporting of currently available molecular tests will need to be reassessed in the era of NIFTP. Early work confirms that NIFTP terminology will alter test specificity and overall rate of malignancy, essentially by decreasing the positive predictive value of the test (44). For this reason, Thyroseq® results are now reported as being either positive or negative for a mutation, and a probability of cancer including its type and aggressiveness is given according to the type of alteration, in order to better assist management. In contrast to Thyroseq®, Afirma® is less informative since it only provides a binary result (benign vs. suspicious) rather than a specific genetic alteration (42). Limited studies suggest that NIFTP can be detected by Afirma® classifier, with most NIFTP being classified as “suspicious”, and that it actually represents the most common “carcinoma” associated with a suspicious result (42,43).

Full table
Ultrasonographic features of NIFTP
US risk stratification of thyroid nodules with indeterminate cytology is addressed separately in this focused issue. Herein, we briefly review the salient features of NIFTP.
Yang et al. have reviewed 179 cases and have categorized ultrasonography characteristics and cytomorphologic FNA features for different FVPTC categories (45). They found that the US features of NIFTP and minimally invasive EFVPTCs were similar and could not be distinguished from each other; both typically exhibited a circumscribed oval or round nodule with a hypoechoic rim and a hypervascular Doppler signal. In contrast, US features for overtly invasive EFVPTC typically showed a round or oval nodule with irregular margins and hypervascularity on Doppler. US for an invasive FVPTC typically reveals at least one of these features: markedly hypoechoic, taller-than-wide, microcalcifications, or blurred margins with an avascular Doppler pattern. US features of NIFTP appear to be most often similar to follicular adenoma. Most but not all NIFTPs are benign-appearing, round-to-oval, circumscribed nodules with a hypoechoic rim, without microcalcifications on US.
Recently, the American College of Radiology (ACR) proposed a Thyroid Imaging Reporting and Data System (TI-RADS) for thyroid nodules based on ultrasonographic features. In the study by Rosario et al. (46), nodules corresponding to NIFTP were classified according to ACR as TI-RADS 3 in 28.5% of cases, TI-RADS 4 in 67.8%, and TI-RADS 5 in only 3.5%. In contrast, nodules corresponding to cancer were classified as TI-RADS 3 in only 2.3% of cases, TI-RADS 4 in 27%, and TI-RADS 5 in 70.5%.
Therefore, US features appear to complement cytology findings and may help raise pre-operative concern for NIFTP in the proper clinical setting, potentially leading to a more conservative management approach.
Coming to terms with NIFTP on cytology; the revised Bethesda system 2017
In 2017, the NCI published a revision and update on the Bethesda system for reporting for thyroid cytopathology (21) based mainly on the symposium entitled “The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC): Past, Present, and Future” held at the October 2016 International Congress of Cytology in Yokohama, Japan, the results of which were published in a joint publication summarizing the proposed modifications and updates for the second edition from an international panel (47,48). The 2017 Bethesda System revision was inspired by new data and new developments in the field of thyroid pathology since 2009, including not only NIFTP terminology (2) but also revised guidelines for the management of patients with thyroid nodules (7) and the introduction of molecular testing as an adjunct to cytopathologic examination.
Since NIFTP cannot be accurately diagnosed preoperatively, the main challenge is how to provide the surgeon with reliable and useful information before the histological diagnosis in order to optimally guide the initial surgical planning, with the main objective of avoiding overtreatment for an indolent neoplasm. To this purpose, several adjustments were made in order to accommodate NIFTP concept on cytology. In TBSRTC 2017 (21), the six general diagnostic categories remained unchanged. ROMs have been updated based on data since 2010 and are calculated in two ways: when NIFTP is not considered a malignancy and when NIFTP is still included among carcinomas (see above). Molecular testing was incorporated as an option for usual management of AUS/FLUS and FN/SFN nodules. Additionally, the diagnostic criteria for FN/SFN and malignancy-PTC were slightly modified (49).
Modified diagnostic criteria
Because of the subsequent overtreatment and potential medico-legal implications, it is highly desirable to exclude potential NIFTP cases from the Malignant category, and to limit the malignant category to conventional PTC and other variants of PTC (21,49,50). The clinical impact of NIFTP is not confined to the malignant category, as many patients with an SFM cytology diagnosis are also treated with an upfront total thyroidectomy, making the drop in ROM for the SFM category also important. In a survey conducted in 2015, prior to the NIFTP reclassification of 2016 and the revised more conservative ATA guidelines of 2015, among clinically active members of the Endocrine Society, ATA and American Association of Clinical Endocrinologists, 92% of respondents indicated that they performed a total thyroidectomy based on a malignant FNA diagnosis, and 43% reported performing an initial total thyroidectomy if the FNA diagnosis is SFM (51).
It is now suggested in the second edition of TBSRTC (21,49) that a definitive diagnosis of PTC (Malignant) should be limited to cases that have, in addition to other characteristic features, at least one of the following: papillary architecture, psammoma bodies, or several nuclear pseudoinclusions. An aspirate displaying only microfollicles with PTC nuclear features, even when obvious, should raise concern for NIFTP. However, it is unlikely that NIFTP can be eliminated entirely from the malignant category. For example, rare cases of NIFTP have frequent pseudoinclusions and, thus, these cases might be categorized as malignant on FNA despite more strict criteria. As a result, it is important for clinicians and patients to be aware that, although the vast majority of nodules with a malignant diagnosis on FNA will be a PTC on resection, there is still a low rate of false positive results due to some tumors which are related to PTC and share their nuclear features such as hyalinizing trabecular tumor and now NIFTP. Rather than creating a separate diagnostic category to accommodate NIFTP, having most NIFTP cases diagnosed in the FN/SFN category, which was historically designed to identify potential FTC, make sense, since the management of these neoplasms is similar (47,48).
Explanatory notes with NIFTP in the differential diagnosis (“NIFTP note”)
An option initially suggested by Krane et al. (50) which has been endorsed by TBSRTC (21,47-49) is to add explanatory notes to the cytologic diagnosis about the possibility of NIFTP on histologic follow-up. This can be particularly useful to support a more conservative clinical management as recommended by the latest ATA guidelines for low-risk thyroid neoplasms (7,17) (see below).
What is the impact on the management?
Surgical management of cytologically indeterminate thyroid nodules is addressed separately in this focused issue. Currently, most but not all of the authorities consider NIFTP as a surgical disease, which requires resection for diagnostic purpose and to prevent possible progression to invasive phenotype (5,7,17). As clinical management of these tumors is being evaluated by various professional societies, further recommendations for managing patients with NIFTP and other borderline tumors are expected to be issued and incorporated into clinical practice. As per current ATA practice guidelines (7,17), the proposed reclassification/terminology change is primarily semantic in nature. However, the proposed reclassification should not be interpreted as indicative of a changed risk profile of an inherently low-risk neoplasm, or as supporting a nonsurgical approach to these neoplasms, as accurate preoperative identification of NIFTP has not yet been demonstrated (17). NIFTP lesions nonetheless warrant excision by lobectomy to exclude an invasive FVPTC, classical PTC, or other thyroid malignancy (5). Once the diagnosis of NIFTP is made histologically, however, further therapy such as completion thyroidectomy and/or radioactive iodine therapy may not be warranted and a more conservative approach is recommended (17). The full impact of NIFTP is yet to be determined but it will further encourage an initial lobectomy in many circumstances.
With NIFTP concept and the revised ATA guidelines (7,17), the potential role of frozen section (intraoperative consultation) to guide the extent of surgery (i.e., total thyroidectomy, lymph node dissection) for cases suspicious for NIFTP on cytology and for indeterminate thyroid cytology in general (Bethesda III–V) needs to be reevaluated by prospective studies. Due to limited sampling, a definitive diagnosis of NIFTP is not possible during intraoperative consultation. Akin to FTC, limited sampling during frozen section is also unlikely to identify minimal capsular or vascular invasion and to distinguish invasive EFVPTC from NIFTP. Also, manipulating/cutting the fresh (unfixed) nodule during intraoperative exam can hinder the definitive analysis of the tumor capsule and the identification of subtle foci of capsular and vascular invasion. In contrast, it may be possible to rule out NIFTP by its exclusion criteria (e.g., invasion or papillary architecture) and to recognize the invasive FVPTC or classical PTC with predominant follicular growth based on their different architectural features. However, as for molecular tests, this would be useful only if the results are expected to change the extent of surgery.
In contrast to this Western approach, the active surveillance for indeterminate nodules and NIFTP, largely represented in the indeterminate cytologic categories which is endorsed by Japanese institutions establishes an alternative model to reduce overtreatment of these patients (27). According to the Japan Thyroid Association clinical guidelines, diagnostic surgery is not indicated for patients with FN/SFN nodules when patients have benign clinical findings. However, patients with FN/SFN nodules are actively monitored until any suspicious clinical and/or imaging findings appear (27).
Limitations and future directions
The NIFTP concept is recent and is still a work in progress. The indolent behavior, natural history, optimal treatment and follow-up of this disease should be further addressed in additional studies, preferably in prospective series of patients with long-term follow-up (52,53). Other studies will also allow need to address the reproducibility and robustness of the diagnostic criteria for NIFTP.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Masson P. Human Tumors. 2nd edition. Detroit: Wayne State University Press, 1970.
- Nikiforov YE, Sethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016;2:1023-9. [Crossref] [PubMed]
- Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to noninvasive follicular thyroid neoplasm with papillary like nuclear features would help to prevent overtreatment. Mod Pathol 2016;29:698-707. [Crossref] [PubMed]
- Baloch ZW, Seethala RR, Faquin WC, et al. Noninvasive follicular thyroid neoplasm with papillary like nuclear features (NIFTP): a changing paradigm in thyroid surgical pathology and implications for thyroid cytopathology. Cancer Cytopathol 2016;124:616-20. [Crossref] [PubMed]
- Ferris RL, Nikiforov Y, Terris D, et al. AHNS Series: Do you know your guidelines? AHNS Endocrine Section Consensus Statement: State-of-the-art thyroid surgical recommendations in the era of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Head Neck 2018;40:1881-8. [Crossref] [PubMed]
- Armstrong MJ, Yang H, Yip L, et al. PAX8/PPARγ rearrangement in thyroid nodules predicts follicular-pattern carcinomas, in particular the encapsulated follicular variant of papillary carcinoma. Thyroid 2014;24:1369-74. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Tallini G, Tuttle RM, Ghossein RA. The History of the Follicular Variant of Papillary Thyroid Carcinoma. J Clin Endocrinol Metab 2017;102:15-22. [PubMed]
- Tallini G, Tuttle RM, Ghossein RA. The History of the Follicular Variant of Papillary Thyroid Carcinoma. J Clin Endocrinol Metab 2017;102:15-22. [PubMed]
- Elsheikh TM, Asa SL, Chan JK, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol 2008;130:736-44. [Crossref] [PubMed]
- Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria and Explanatory Notes. New York, NY: Springer, 2009.
- Daniels GH. Follicular Thyroid Carcinoma: A Perspective. Thyroid 2018;28:1229-42. [Crossref] [PubMed]
- Nikiforov YE, Baloch ZW, Hodak SP, et al. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillary like nuclear features. JAMA Oncol 2018;4:1125-6. [Crossref] [PubMed]
- Alves VA, Kakudo K. Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features (NIFTP): Achieving Better Agreement By Refining Diagnostic Criteria. Clinics (Sao Paulo) 2018;73:e576. [Crossref] [PubMed]
- Seethala RR, Baloch ZW, Barletta JA, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists. Mod Pathol 2018;31:39-55. [Crossref] [PubMed]
- Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary like nuclear features. Thyroid 2017;27:481-3. [Crossref] [PubMed]
- Lloyd RV, Osamura RY, Klöppel G, et al. WHO Classification of Tumours of Endocrine Organs, 4th edition, Volume 10, 2017.
- Bychkov A, Jung CK, Liu Z, et al. Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice: Perspectives for Surgical Pathology and Cytopathology. Endocr Pathol 2018;29:276-88. [Crossref] [PubMed]
- Bychkov A, Hirokawa M, Jung CK, et al. Low Rate of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice. Thyroid 2017;27:983-4. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes. 2nd edition. New York, NY: Springer, 2017.
- Faquin WC, Wong LQ, Afrogheh AH, et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol 2016;124:181-7. [Crossref] [PubMed]
- Pusztaszeri MP, Triponez F, Meyer P, et al. Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features (NIFTP): Report of an Institutional Experience with 86 Cases. J Basic Clin Med 2017;6:29-35.
- Strickland KC, Howitt BE, Marqusee E, et al. The impact of noninvasive follicular variant of papillary thyroid carcinoma on rates of malignancy for fine needle aspiration diagnostic categories. Thyroid 2015;25:987-92. [Crossref] [PubMed]
- Howitt BE, Chang S, Eslinger M, et al. Fine-needle aspiration diagnoses of noninvasive follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2015;144:850-7. [Crossref] [PubMed]
- Zhou H, Baloch ZW, Nayar R, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): implications for the risk of malignancy (ROM) in The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). Cancer Cytopathol 2018;126:20-6. [Crossref] [PubMed]
- Kakudo K, Higuchi M, Hirokawa M, et al. Thyroid FNA cytology in Asian practice-Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017;28:455-66. [Crossref] [PubMed]
- Brandler TC, Zhou F, Liu CZ, et al. Can noninvasive follicular thyroid neoplasm with papillary-like nuclear features be distinguished from classic papillary thyroid carcinoma and follicular adenomas by fine-needle aspiration? Cancer Cytopathol 2017;125:378-88. [Crossref] [PubMed]
- Strickland KC, Vivero M, Jo VY, et al. Preoperative cytologic diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a prospective analysis. Thyroid 2016;26:1466-71. [Crossref] [PubMed]
- Bizzarro T, Martini M, Capodimonti S, et al. The morphologic analysis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on liquid based cytology: some insights of their identification in our institutional experience. Cancer Cytopathol 2016;124:699-710. [Crossref] [PubMed]
- Ibrahim AA, Wu HH. Fine-Needle Aspiration Cytology of Noninvasive Follicular Variant of Papillary Thyroid Carcinoma Is Cytomorphologically Distinct From the Invasive Counterpart. Am J Clin Pathol 2016;146:373-7. [Crossref] [PubMed]
- Maletta F, Massa F, Torregorssa L, et al. Cytological features of “non-invasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol 2016;54:134-42. [Crossref] [PubMed]
- Zhao L, Dias-Santagata D, Sadow PM, et al. Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer Cytopathol 2017;125:323-31. [Crossref] [PubMed]
- Pusztaszeri M, Auger M. Updates on the cytologic features of papillary thyroid carcinoma variants. Diagn Cytopathol 2017;45:714-30. [Crossref] [PubMed]
- Renshaw AA, Gould EW. Incidence and significance of true papillae in thyroid fine needle aspiration. Diagn Cytopathol 2017;45:689-92. [Crossref] [PubMed]
- Strickland KC, Eszlinger M, Paschke R, et al. Molecular Testing of Nodules with a Suspicious or Malignant Cytologic Diagnosis in the Setting of Non-Invasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features (NIFTP). Endocr Pathol 2018;29:68-74. [Crossref] [PubMed]
- Strickland KC, Howitt BE, Barletta JA, et al. Suggesting the cytologic diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): A retrospective analysis of atypical and suspicious nodules. Cancer Cytopathol 2018;126:86-93. [Crossref] [PubMed]
- Onenerk AM, Pusztaszeri M, Canberk S, et al. Triage of the Indeterminate Thyroid Aspirate: What are the Options for the Practicing Cytopathologist? Cancer Cytopathol 2017;125:477-85. [Crossref] [PubMed]
- Jiang XS, Harrison GP, Datto MB. Young investigator challenge:molecular testing in noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Cancer Cytopathol 2016;124:893-900. [Crossref] [PubMed]
- Cho U, Mete O, Kim MH, et al. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol 2017;30:810-25. [Crossref] [PubMed]
- Song YS, Won JK, Yoo SK, et al. Comprehensive Transcriptomic and Genomic Profiling of Subtypes of Follicular Variant of Papillary Thyroid Carcinoma. Thyroid 2018;28:1468-78. [Crossref] [PubMed]
- Jug RC, Datto MB, Jiang XS. Molecular testing for indeterminate thyroid nodules: Performance of the Afirma gene expression classifier and ThyroSeq panel. Cancer Cytopathol 2018;126:471-80. [Crossref] [PubMed]
- Brandler TC, Liu CZ, Cho M, et al. Does Noninvasive Follicular Thyroid Neoplasm With Papillary-Like Nuclear Features (NIFTP) Have a Unique Molecular Profile? Am J Clin Pathol 2018;150:451-60. [Crossref] [PubMed]
- Sahli ZT, Umbricht CB, Schneider EB, et al. Thyroid Nodule Diagnostic Markers in the Face of the New NIFTP Category: Time for a Reset? Thyroid 2017;27:1393-9. [Crossref] [PubMed]
- Yang GCH, Fried KO. Pathologic basis of the sonographic differences between thyroid cancer and noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Ultrasonography 2018;37:157-63. [Crossref] [PubMed]
- Rosario PW, da Silva AL, Nunes MB, et al. Risk of Malignancy in Thyroid Nodules Using the American College of Radiology Thyroid Imaging Reporting and Data System in the NIFTP Era. Horm Metab Res 2018;50:735-7. [Crossref] [PubMed]
- Pusztaszeri M, Rossi ED, Auger M, et al. The Bethesda system for reporting thyroid cytopathology: proposed modifications and updates for the second edition from an international panel. Acta Cytol 2016;60:399-405.
- Pusztaszeri M, Rossi ED, Auger M, et al. The Bethesda system for reporting thyroid cytopathology: proposed modifications and updates for the second edition from an international panel. J Am Soc Cytopathol 2016;5:245-251.
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017;27:1341-6. [Crossref] [PubMed]
- Krane JF, Alexander EK, Cibas ES, et al. Coming to terms with NIFTP: A provisional approach for cytologists. Cancer Cytopathol 2016;124:767-72. [Crossref] [PubMed]
- Burch HB, Burman KD, Cooper DS, et al. A 2015 Survey of Clinical Practice Patterns in the Management of Thyroid Nodules. J Clin Endocrinol Metab 2016;101:2853-62. [Crossref] [PubMed]
- Parente DN, Kluijghout WP, Bongers PJ, et al. Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma. Is NIFTP truly benign? World J Surg 2018;42:321-6. [Crossref] [PubMed]
- Chereau N, Greilsamer T, Mirallié E, et al. NIFT-P: Are they indolent tumors? Results of a multi-institutional study. Surgery 2019;165:12-6. [Crossref] [PubMed]


