
Optimizing perioperative strategies to maximize success with prepectoral breast reconstruction
Introduction
With a rising incidence of breast cancer, increasing numbers of women are seeking post mastectomy breast reconstruction (1,2). To this day, the most commonly employed reconstructive technique involves the placement of a subpectoral tissue expander with subsequent exchange for an implant in the same anatomic plane (3). This approach requires disinsertion of the pectoralis muscle [with acellular dermal matrix (ADM) as a common adjunct]. Draping of the pectoralis major muscle over the implant results in animation deformity—where the implant and reconstructed breast is distorted with activation of the pectoralis. The skin frequently forms adhesions to the pectoralis muscle, and the results in significant dimpling of the skin with pectoralis activation. Further, the vector of muscle force pulls the implant towards the axilla. Additionally, the subpectoral surgical dissection results in increased postoperative pain often requiring the use of muscle relaxants for symptomatic management of muscle spasm.
Placement of a breast prosthesis in the subcutaneous pocket post mastectomy is not a new-age innovation. In fact, this was the originally described approach to breast reconstruction post mastectomy (4,5). However, this technique was wrought with complications including malposition, infection, contracture, and eventual reconstructive failure. Accordingly, the subpectoral approach became a standard, allowing the pectoralis muscle to add additional coverage over the implant in order to mitigate the frequency of these complications. As reconstructive surgery evolves, adjuncts such as tissue perfusion technology, fat grafting, ADM, and improved mastectomy techniques have allowed for optimization of recipient tissue. These additions have allowed for prepectoral reconstruction to be re-evaluated as a safe, and potentially superior option for many patients receiving post mastectomy breast reconstruction.
As with all operations, patient selection is key. Numerous variables can be screened for pre-operatively, but a notable component of pursuing prepectoral breast reconstruction is careful intra-operative evaluation of the mastectomy skin flaps and breast borders. Once the decision is made to place a prepectoral prostheses, close post-operative follow-up with both anticipation of complications and prompt response are warranted. This article will define strategies pre-operatively, intra-operatively, and post-operatively to optimize outcomes with prepectoral breast reconstruction.
Pre-operative evaluation
General health considerations
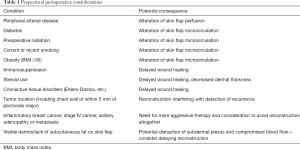
As with any surgical preoperative evaluation, general health considerations including a full history and physical are necessary. In a general sense, patients should be healthy enough to undergo general anesthesia for multiple operations. National Surgical Quality Improvement Program (NSQIP) recommendations for pre-operative electrocardiogram (EKG) and echocardiogram should be followed, and peri-operative risks identified and optimized. Successful breast reconstruction, specifically pre-pectoral reconstruction, requires adequate wound healing and sufficient flap perfusion. Accordingly, medical comorbidities should be documented, and those affecting wound healing and vascular supply should give pause when considering prepectoral breast reconstruction. Table 1 reviews vascular and connective tissue disorders that are not contraindications, rather, require special consideration in the setting of prepectoral implant placement. Additionally, higher risks for complications in patients with diabetes mellitus, obesity, and tobacco use should be discussed and documented. For those patients with diabetes, it is necessary to confirm that blood glucose levels are within normal limits, and under strict control. A recent Hemoglobin A1c (within the last 3 months) should be less than 7%. Higher values indicate room for improvement in glycemic control. Furthermore, active smokers should not be offered prosthetic reconstruction and should be tobacco-free for at least 3 months prior to surgery. A urine cotinine level can be obtained to confirm smoking cessation prior to surgery.
Full table
Oncologic management
Breast cancer treatment is now particularly multidisciplinary, with receptor specific chemotherapies, changing radiation protocols, and neoadjuvant exposure to treatments in order to optimize extirpative results. Patients are presenting for reconstruction at various stages in treatment, and it is incumbent on the reconstructive surgeon to evaluate breast reconstruction options specific to each patient’s cancer treatment.
Radiation
Radiation therapy usually entails whole breast radiation, with potential boost doses to specific areas based on tumor location and size. The deleterious effects of radiation on wound healing are well described, and of specific concern in implant based reconstruction.
Prior breast radiation
Patients who have already undergone radiation therapy should be evaluated for the degree of damage sustained. Since the significant inflammatory response to radiation lingers for many months after completion of treatment, we recommend waiting a minimum of 6 months before considering delayed reconstruction in these patients. However, increased wound healing complications, including implant exposure and failure of implant based reconstruction, categorizes these patients as suboptimal candidates for any type of prosthetic reconstruction, including prepectoral placement (6). Autologous fat grafting has been proposed as an adjunct to optimize tissue and minimize radiation damage prior to implant placement in delayed reconstruction (7). Ideally, autologous tissue reconstruction should be offered to mitigate these wound healing risks. However, when absolutely necessary, prepectoral reconstruction can be performed on these patients, and likely a superior option to subpectoral reconstruction in this same cohort. The radiated muscle becomes particularly fibrotic, and translates additional force on to the implant. This can result in implant malposition and capsular contracture.
Post-mastectomy radiation therapy (PMRT)
For those patients who will need PMRT, we recommend immediate reconstruction, allowing for expansion and possibly implant exchange prior to PMRT. Previous studies suggest that overall aesthetic results may be suboptimal with expander radiation, but that long-term capsular contracture rates are significantly better with expander radiation compared to permanent implant radiation (8). Short-term studies suggest that PMRT does not increase the risk of adverse outcomes with prepectoral reconstruction, and further, in comparison to subpectoral placement, some authors suggest that prepectoral placement significantly decreases the risk of capsular contracture when PMRT is necessary (9,10). The authors have found that prepectoral devices migrate less during PMRT, relative to subpectoral devices, due to the lack of a contracting muscle pulling the prosthesis as it undergoes fibrosis during radiation. As such, prepectoral reconstruction allows for more predictable aesthetic outcomes following radiation, as the soft tissue envelope dimensions remain where they were surgically placed through radiation and after.
Tumor specific considerations
The location, and distance from chest wall of the tumor, is also significant. Tumors that are palpable may require an additional or atypical skin resection to appropriately obtain clear margins. Based on the location of the tumor, these skin resections may extend outside of standard incisions. This can potentially distort the pocket size and implant position on the chest wall, and should be discussed with the patient pre-operatively to best manage expectations and any need for revisions. Further, patients with tumors within 5 mm of the pectoralis fascia, or known chest wall invasion, are not candidates for prepectoral breast reconstruction (11). These patients are at higher risk for recurrence, and placement of a prepectoral implant can delay detection of any recurrences. Finally, patients with inflammatory carcinoma should not be offered prepectoral reconstruction due to the lymphatic obstruction in areas of their skin envelope, which is exacerbated after mastectomy (12,13).
Physical exam
In depth examination of the whole patient, and of course bilateral breasts and axilla, are necessary pre-operative measures for every implant-based breast reconstruction patient. Furthermore, intra-operative evaluation of skin flap viability is also crucial in decision making and timing of reconstruction.
Pre-operative examination
Pre-operative physical examinations should be thorough. Every breast exam should include an evaluation for masses, lumps, previous scars, nipple discharge, skin quality, and tissue volume. Specific to each breast, standard measurements should be obtained in order to objectively document any asymmetries. These measurements should include the distance from the sternal notch to each nipple, each nipple to the inframammary fold (IMF), and inter-nipple distance. Breast width should also be noted as this becomes especially relevant when choosing a final implant size. In addition to this, visual inspection should note any asymmetries in size (especially when patients have had previous breast surgery and/or radiation), projection, chest wall position, and ptosis. Skin quality is of specific importance with prepectoral implant placement because there is no additional muscle coverage. Therefore, reasonable thickness to the skin with moderate subcutaneous fat is favorable. Breast size is important in that larger, pendulous breasts tend to rely heavily on the ADM sling to support the implant. Small to moderate sized breasts are ideal for prepectoral reconstruction, however with an adequate skin envelope, larger implants can be used safely.
Patient selection and informed consent
The aforementioned considerations for pre-operative evaluation all aim to answer the main question: is this patient a good candidate for prepectoral breast reconstruction? The advantages are clear: reduced short- and long-term pain and avoidance of animation deformity. However, without the added muscle coverage over the implant, we more heavily rely on wound healing and flap quality. As such, the main contraindications for prepectoral breast reconstruction include devascularized skin envelope, uncontrolled diabetes mellitus, recent or current tobacco use, and tumor location within 5 mm of the pectoralis fascia. Moderately controlled diabetes mellitus, vascular disease, and obesity [body mass index (BMI) >35] warrant further discussion regarding increased risks with the patient, but are not true contraindications.
The authors advocate that each patient be consented for prepectoral, subpectoral and delayed reconstruction, in all cases. It is the author’s practice to make the final decision on reconstructive choice in the operating room, following completion of mastectomy, based on which option is safest at that time. As such, all the options are discussed with each patient preoperatively.
Multidisciplinary care benefits
As with all reconstruction, open communication with the surgical oncologist is beneficial in surgical planning. The primary goal is to perform sound oncologic surgery. However, a variety of mastectomy techniques have emerged, all with their own indications, benefits, and risks, that have been deemed safe alternatives in the appropriate patient. Generally speaking, the decision to perform nipple sparing vs. skin sparing vs. simple mastectomies depends on necessary oncologic margins and surgeon comfort. Prepectoral reconstruction can be performed through any available incisions, but patient specific variables (skin quality, tumor location, breast size, patient preference) should be considered in deciding which incision is optimal. Preoperative markings should be agreed upon by both surgical teams and confirmed with the patient.
Prepectoral direct to implant (DTI) reconstruction
A small subset of patients may qualify for DTI reconstruction. The indications for prepectoral DTI are the same as subpectoral DTI. Patients need to have optimal skin quality, excellent mastectomy flap perfusion, mild ptosis, and be of mild to moderate breast size. Although long-term outcomes with prepectoral DTI reconstruction have not been studied extensively, smaller studies suggest similar outcomes to subpectoral DTI with respect to complications and implant loss (14).
Prepectoral conversion of a subpectoral implant
Indications for prepectoral conversion of a subpectoral implant are two-fold: capsular contracture, and animation deformity. The considerations for candidacy are the same as mentioned above for immediate reconstruction. Any patient presenting with post mastectomy reconstruction pain should be evaluated for all etiologies, including post mastectomy pain syndrome which is more neuropathic in etiology. This is important to document because with prepectoral revision, capsular scar is removed and associated pain should resolve, but other sources of pain may not. Techniques for prepectoral conversion will be covered in a separate chapter in this issue.
Informed consent
Patients undergoing prepectoral placement of expanders and implants should be adequately counseled on the following risks:
- early complications
- anesthesia risk including intra-operative cardiopulmonary risks;
- deep vein thrombosis (DVT)/pulmonary embolus (PE);
- seroma;
- hematoma;
- superficial and deep infections;
- implant loss;
- late complications
- capsular contracture (although studies show lower incidence with prepectoral placement) (15);
- implant rupture: silicone implant monitoring recommendations by the FDA (3 years post placement, and every 2 years to follow);
- implant malposition;
- need for future revisions;
- if textured implants are used, ALCL should also be discussed, with a lifetime risk of 1:10,000–1:30,000 for textured implants (16).
Intraoperative considerations
Mastectomy flap evaluation
Post-mastectomy flap evaluation is a key component in the decision making for implant based immediate breast reconstruction. Especially with prepectoral implant placement, the mastectomy flaps and ADM will be the only layers providing coverage to the implant. If mastectomy skin necrosis occurs, implant exposure and failure of the reconstruction are likely.
Visual inspection should include evaluation of adequate tissue perfusion by way of warmth, color, and capillary refill. Visible bleeding at all cut edges is necessary. If concern arises, indocyanine green angiography can be utilized for real time feedback on tissue perfusion (17). Further, the surgeon should evaluate for any breaks in the skin, exposed dermis on the underside of the flap, and paucity of subcutaneous fat, as predictors of flap quality and viability. Loss of subcutaneous fat with exposed dermis indicates compromise to the subdermal plexus, the blood supply necessary for mastectomy skin viability. Concerning flaps should be closed primarily, with plans for delayed reconstruction. Adjunct fat grafting prior to delayed expander placement in these cases can augment subcutaneous fat and bulk mastectomy flap tissue (18).
When prepectoral tissue expanders are placed at the time of mastectomy, it is often advisable to fill with minimal saline or leave the expander unfilled. This is especially important in patients with perfused, but thin skin flaps. This underfilling of expanders allows for less stretch on the overlying skin flaps and less chance of inducing venous congestion. Careful attention should be paid to the perfusion of skin flaps if immediate filling is performed to minimize the risk of skin flap ischemia (11).
The boundaries of the breast
The breast surgeons aim to remove breast tissue for the purpose of oncologic safety, this sometimes includes a dissection beyond the borders of the breast. Visual inspection and manual palpation are key to confirm that the IMF, medial and lateral borders, and cephalad border of the breast are maintained. The lateral and medial borders can be adjusted with appropriate placement of the ADM sling, and therefore violation of these borders can be readily rectified. With respect to the medial border, violation of the adherent tissue overlying the sternum can lead to symmastia. This can be very difficult to repair once violated. As such, in this setting, subpectoral placement is likely a better option because medial elevation of the pectoralis muscle can be modified to help maintain and re-establish the medial boundary. The IMF is also difficult to re-establish once violated. As with subpectoral placement, the ADM sling can be precisely sutured to the desired IMF to create the inferior boundary. However, since this supports most of the weight of the implant, the natural IMF should also be maintained to avoid inferior migration of the implant. In cases where this border is violated, internal retention sutures or an external approach (Ryan procedure) may be utilized. Since ADM defines this border regardless of subpectoral or prepectoral implant placement, violation of the IMF is not a contraindication to proceeding with prepectoral breast reconstruction.
Post-operative considerations
Infection
Pre-operative antimicrobial measures should be employed and protocoled in all implant patients. First, all patients should have a nasal swab for MRSA. If positive, topical mupirocin is recommended for decolonization (19). Hibiclens showers in all patients the night before surgery is also recommended. Also, preoperative administration of IV Kefzol (and IV Clindamycin in patients with allergies) within 60 minutes of incision is warranted to minimize the risk of surgical site infections (SSI).
Both superficial and deep SSIs require immediate attention given the risk for implant infection. Biofilms often result in persistent infections difficult to eradicate, even with prolonged intravenous antibiotics. Bennett et al. recently published in a multicenter prospective study indicating a 10–15% wound infection rates for DTI and expander to implant reconstruction. Additionally, these same cohorts had a 7.1% reconstructive failure rate (20). Our group has published similar outcomes, with approximately 15% total infectious complication rate, albeit a lower explantation rate. This same series showed no significant difference between the subpectoral and prepectoral cohorts (21). Larger series and longer-term studies are still warranted for more definitive data on whether the plane of dissection for implant placement affects infection rates. Regardless, without at least partial muscular coverage, breakdown of skin will likely lead to implant exposure. Therefore, all infections should be managed aggressively with antibiotics and close follow-up. Any threatened implant exposure demands immediate and proactive skin debridement, removal of the implant, and intravenous antibiotics. There is no consensus on whether an implant should be replaced immediately, or in a delayed fashion. Regardless, clinical judgment is key and no implant should be replaced in to an obviously contaminated and infected field.
Postoperative dressings
Skin closure should be reinforced with a water tight dressing, either steri-strips or skin glue. Overlying this, the application of a large gauze dressing covered with Tegaderm is beneficial. This adds light pressure to the skin flaps, promoting adherence to the underlying ADM. The use of Biopatch dressings at all drain sites and coverage with water-tight Tegaderm works to minimize contamination of the drains, and potential entry point for infection into the surgical site. Dressings can be removed at the first post-operative visit.
Seroma
Multiple studies have confirmed the higher incidence of seromas with the use of ADM (22,23). The utilization of ADM in prepectoral reconstruction for total implant coverage necessitates the placement of two drains in most cases, to account for the increased drainage. Because of ADM fenestration, both drains may be placed between the ADM and overlying skin. Further, although no prospective trials have been performed, previous authors have recommended maintaining the drains in place for a minimum for 2 weeks to capture this additional drainage as the ADM incorporates (11).
Capsular contracture
Multiple studies have shown decreased rates of capsular contracture with prepectoral implant placement. Further, with PMRT, although capsular contracture occurs in greater incidence in both prepectoral and subpectoral implants, the prepectoral cohort is seemingly less affected (9,10). Regardless, capsular contracture is still a potential adverse outcome, and patients should be counseled on signs and symptoms. Treatment is the same as with subpectoral implants.
Montelukast (leukotriene antagonist) has been discussed as a potential treatment for Baker 1 and 2 capsular contracture, but there is a paucity of long-term and high-quality data. Montelukast has also been discussed in the prophylactic setting, but again, studies are low volume with short-term follow-up (24,25). Capsulectomy with implant replacement is still considered the surgical treatment of choice for symptomatic patients, but a certain cohort will still experience recurrence of symptoms despite surgical intervention. To our knowledge, no studies have been performed regarding management and outcomes of capsular contracture specifically in prepectoral reconstruction patients.
Aesthetic outcomes
Implant migration and rippling have both been reported with prepectoral placement of breast implants, albeit larger series with longer term follow-up are necessary to say definitively whether the incidence is different when compared to subpectoral placement. Technique will be discussed in subsequent chapters, but select management strategies may ameliorate some of these issues. During expander filling, underfilling the expander (by ~100–200 cc) relative to anticipated final implant size allows for the final implant to be placed into a tighter pocket, thereby reducing mobility of the implant and visible rippling due to excess and redundant upper pole skin. Further, implant selection is important. The use of silicone implants (as opposed to saline implants) will decrease the amount of visible rippling (26). As an alternative, overfilling saline implants can also decrease rippling post-operatively. It is advisable to use filled to capacity gel implants, and cohesive gel implants, both of which are more rippling resistant. Post-operative revisions should also be discussed with the patient, as they are often a necessary adjunct to optimize outcomes. Fat grafting the mastectomy skin flap can also camouflage the rippling, especially in the upper pole of the breast (11). Although we routinely perform upper pole capsulotomies to aid in redraping of the upper pole skin, this transition can still be subject to a palpable or visible “shelf”, or rippling. We routinely fat graft this upper pole skin at the time of expander replacement to camouflage these findings. In our experience, at least two-thirds of our patients benefit from fat grafting, although this does not seem to be different from the total number of patients with subpectoral implants that also benefit from fat grafting revisions. We discuss this adjunct procedure preoperatively with the patient, and include it on all consents for patients undergoing expander replacement with the permanent implant. Therefore, we can evaluate intra-operatively and fat graft as needed. This also avoids an additional surgery at a later time.
Conclusions
Prepectoral breast reconstruction has emerged as a valid alternative to traditional subpectoral implant placement for post mastectomy breast reconstruction. Prosthesis placement above the pectoralis muscle avoids animation deformities and decreases post-operative pain. However, successful pre-pectoral reconstruction hinges on adequately perfused mastectomy flaps and the use of an ADM sling.
Multiple strategies exist to optimize outcomes from prepectoral breast reconstruction. Appropriate patient selection and minimizing risk factors are integral to success. Mastectomy skin flaps should be evaluated for quality after extirpative surgery. Implant selection can also affect rippling and the risk for implant malposition. Post-operatively, close monitoring for surgical complications is vital.
Prepectoral breast reconstruction offers multiple benefits in the appropriate patient. Reconstructive surgeons should employ distinct criteria in patient selection, mastectomy flap evaluation, intraoperative techniques and postoperative monitoring to optimize results.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Sbitany is a consultant for Allergan, Inc. The other authors have no conflicts of interest to declare.
References
- Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol 2014;32:919-26. [Crossref] [PubMed]
- Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg 2013;131:15-23. [Crossref] [PubMed]
- Sbitany H, Sandeen SN, Amalfi AN, et al. Acellular dermis-assisted prosthetic breast reconstruction versus complete submuscular coverage: a head-to-head comparison of outcomes. Plast Reconstr Surg 2009;124:1735-40. [Crossref] [PubMed]
- Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg 1982;69:195-208. [Crossref] [PubMed]
- Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg 1971;47:565-7. [Crossref] [PubMed]
- Bettinger LN, Waters LM, Reese SW, et al. Comparative Study of Prepectoral and Subpectoral Expander-Based Breast Reconstruction and Clavien IIIb Score Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [Crossref] [PubMed]
- Salgarello M, Visconti G, Barone-adesi L. Fat grafting and breast reconstruction with implant: another option for irradiated breast cancer patients. Plast Reconstr Surg 2012;129:317-29. [Crossref] [PubMed]
- Cordeiro PG, Albornoz CR, Mccormick B, et al. What Is the Optimum Timing of Postmastectomy Radiotherapy in Two-Stage Prosthetic Reconstruction: Radiation to the Tissue Expander or Permanent Implant? Plast Reconstr Surg 2015;135:1509-17. [Crossref] [PubMed]
- Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral Implant-Based Breast Reconstruction and Postmastectomy Radiotherapy: Short-Term Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1631. [Crossref] [PubMed]
- Sinnott CJ, Persing SM, Pronovost M, et al. Impact of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction. Ann Surg Oncol 2018;25:2899-908. [Crossref] [PubMed]
- Sbitany H. Important Considerations for Performing Prepectoral Breast Reconstruction. Plast Reconstr Surg 2017;140:7S-13S. [Crossref] [PubMed]
- Mohamed MM, Al-Raawi D, Sabet SF, et al. Inflammatory breast cancer: New factors contribute to disease etiology: A review. J Adv Res 2014;5:525-36. [Crossref] [PubMed]
- Hashmi S, Zolfaghari L, Levine PH. Does secondary inflammatory breast cancer represent post-surgical metastatic disease? Cancers (Basel) 2012;4:156-64. [Crossref] [PubMed]
- Jones G, Yoo A, King V, et al. Prepectoral Immediate Direct-to-Implant Breast Reconstruction with Anterior AlloDerm Coverage. Plast Reconstr Surg 2017;140:31S-8S. [Crossref] [PubMed]
- Bettinger LN, Waters LM, Reese SW, et al. Comparative Study of Prepectoral and Subpectoral Expander-Based Breast Reconstruction and Clavien IIIb Score Outcomes. Plast Reconstr Surg Glob Open 2017;5:e1433. [Crossref] [PubMed]
- Clemens MW, Miranda RN, Butler CE. Breast Implant Informed Consent Should Include the Risk of Anaplastic Large Cell Lymphoma. Plast Reconstr Surg 2016;137:1117-22. [Crossref] [PubMed]
- Griffiths M, Chae MP, Rozen WM. Indocyanine green-based fluorescent angiography in breast reconstruction. Gland Surg 2016;5:133-49. [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation using Magnetic Resonance Imaging. Plast Reconstr Surg Glob Open 2017;5:e1439. [Crossref] [PubMed]
- Hart A, Desai K, Yoo J, et al. Incidence of Methicillin-Resistant Staphylococcus aureus (MRSA) Carrier Status in Patients Undergoing Post-Mastectomy Breast Reconstruction. Aesthet Surg J 2017;37:35-43. [Crossref] [PubMed]
- Bennett KG, Qi J, Kim HM, et al. Comparison of 2-Year Complication Rates Among Common Techniques for Postmastectomy Breast Reconstruction. JAMA Surg 2018;153:901-8. [Crossref] [PubMed]
- Baker BG, Irri R, Maccallum V, et al. A Prospective Comparison of Short-Term Outcomes of Subpectoral and Prepectoral Strattice-Based Immediate Breast Reconstruction. Plast Reconstr Surg 2018;141:1077-84. [Crossref] [PubMed]
- Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg 2012;68:346-56. [Crossref] [PubMed]
- Lee KT, Mun GH. A Meta-analysis of Studies Comparing Outcomes of Diverse Acellular Dermal Matrices for Implant-Based Breast Reconstruction. Ann Plast Surg 2017;79:115-23. [Crossref] [PubMed]
- Huang CK, Handel N. Effects of Singulair (montelukast) treatment for capsular contracture. Aesthet Surg J 2010;30:404-8. [Crossref] [PubMed]
- Graf R, Ascenço AS, Freitas Rda S, et al. Prevention of Capsular Contracture Using Leukotriene Antagonists. Plast Reconstr Surg 2015;136:592e-6e. [Crossref] [PubMed]
- Isaac KV, Murphy BD, Beber B, et al. The Reliability of Anthropometric Measurements Used Preoperatively in Aesthetic Breast Surgery. Aesthet Surg J 2016;36:431-7. [Crossref] [PubMed]


