Chylous leak: an unexpected complication after microsurgical breast reconstruction
Chyle is composed of lymphatic fluid and chylomicrons from the gastrointestinal system. Its lymphatic fluid contains protein, white blood cells (WBCs), electrolytes, fat-soluble vitamins, trace elements, and glucose absorbed from the interstitial fluid, to be returned to the systemic circulation (1).
Chyle leak (CL) from iatrogenic thoracic duct injury is a well known serious complication of head and neck surgery (2–8% of neck dissections) (2), incidence after axillary clearance and breast cancer surgery has been reported to reach 0.5% (3). However, only a single report of CL after mastectomy, axillary clearance and implant-based reconstruction exists (4), with no chylous leak complications described after microsurgical flap breast reconstruction.
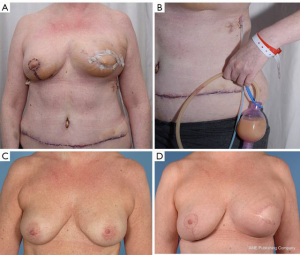
In February 2018, we performed a left sided skin-sparing mastectomy for an invasive ductal carcinoma, associated with axillary clearance and immediate breast reconstruction using a deep inferior epigastric perforator (DIEP) free flap (Figure 1). The usual diagnostic protocol, including mammography, ultrasonography with core-needle biopsy of the nodule, and axillary fine-needle aspiration biopsy was performed. The reconstructive procedure was uneventful, with flap anastomoses to the internal mammary artery and vein. At postoperative day 3, it was noted that the lateral aspect of the reconstructed breast had become erythematous, ‘pus’ was in the drain and two episodes of pyrexia (38 degrees). Blood tests showed WBC of 9.1×109/L and C-reactive protein (CRP) of 232 mg/L.
These levels were indeed compatible with postoperative inflammation but could not exclude formally an infected collection. Considering the pyrexia, an empirical antibiotic treatment (IV flucloxacillin) was started while awaiting definitive microbiology results.
Given the pus-like fluid in the drain, and the increasingly swollen flap, the decision for surgical re-exploration and washout was taken at postoperative day 6, and confirmed a viable flap with healthy bleeding and no signs of fat necrosis. A cytological, microbiological, and biochemical study of the drainage fluid yielded the following: no bacteria seen despite the presence of WBCs, 26 g/L total proteins, 12 g/L albumin, and 8 mmol/L triglycerides with 1.1 mmol/L of cholesterol.
Cut-off values for chylous leak are triglycerides over 1.24 mmol/L or 110 mg/dL (with cholesterol under 5.2 mmol/L or 200 mg/dL), and proteins over 20 g/L (5), making clear that the clinical scenario was consistent with the diagnosis of axillary chylous fistula.
The patient was started on a fat free diet, which allowed for steady decrease in leakage and in triglyceride concentration of the fluid. The milky appearance disappeared and the drain could be removed. After discharge, the patient required a further drainage of an axillary seroma in the outpatient clinic. The wounds healed uneventfully at 6 weeks postoperative follow-up and the patient could receive neoadjuvant radiotherapy with final satisfactory aesthetic and functional outcome (Figure 1).
The thoracic duct is the common final route for most lymphatic fluid, enabling it to return to the blood circulation. The thoracic duct usually drains to the internal jugular vein or subclavian vein but it may join the external jugular vein or innominate vein (6). Classically, the cause of chylous fistula is assumed to be injury to the main thoracic duct or its terminal rami (7). However, an increasing number of authors suggest that the injury is more probably caused at the subclavian duct or its tributaries, as this duct is very inconsistent and its location is closely related to Berg’s level II lymph-node dissection (3). The variable anatomy and fragile composition of the thoracic duct may render it prone to inadvertent injury at this level (8).
The risk factors for the development of axillary lymphatic fistula obviously include surgical technique, but also obesity and potentially early exercise of the shoulder (6).
Chylous fistulas can be divided into low-output (<500 mL/24 h) and high-output (>500 mL/24 h) fistulas. The importance of these fistulas lie in their capacity to compromise the patient’s nutritional and immunological balance, delay wound healing, and lengthen hospital stay (8), which is particularly relevant when complex microsurgical surgery has been performed and when patients require post operative adjuvant therapy. Clinical diagnosis is clear from the timing of the presentation and presence of a milky output in the drainage contents (4). Laboratory analysis comparing serum and chyle lipids and proteins can be useful to confirm clinical suspicion.
A diet low in fat and rich in medium-chain triglycerides, which are absorbed straight into the portal system bypassing the lymphatics and chyle production step, is generally adopted to reduce fistula flow. Supplementary measures include maintaining axillary drainage, a pressure bandage, and rest. Parenteral nutrition was not necessary in our case and should be started only if the leak persists despite dietary modification and initial conservative management (8).
Further contributing to the complexity of chylous leak management is that it may be mistaken for the more common surgical infection after breast reconstruction (infected seromas or hematomas) with typical signs of inflammation—dolor, tumor, calor and rubor or, in cases of autologous reconstruction, flap compromise, flap insufficiency or infected liponecrosis.
In conclusion, chylous leaks are rare but can occur in patients undergoing mastectomies and axillary clearances particularly on the left side. Its diagnosis should be considered if erythema and dense milky drainage occurs in the early post operative period with the commencement of oral diet, in order to avoid unnecessary returns to theatre for washout and re-exploration.
Most chylous fistulas respond well to conservative treatment and resolve in a few days with a low-fat diet. The use of octreotide can be considered, being well documented as a treatment for the more common chylous fistulas of the neck following left-sided lymphadenectomies. This somatostatin analogue seems to reduce lymphatic flow by decreasing the intestinal fat absorption and consequently limiting the flow to the damaged duct, which would make it easier to close off with the usual healing process (9,10).
However, other more aggressive forms of treatment, such as surgical ligation or embolization following lymphangiography, should be considered in the case of high-output or extremely high output fistulas (up to more than 2 L), which persist for several days despite conservative measures. Care should be taken in those with autologous reconstructions especially if the anastomosis is to the thoracodorsal vessels.
Acknowledgements
Authors are grateful to the Medical Illustration Service of the Glasgow Royal Infirmary for their help in clinical pictures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The patient’s written informed consent was obtained for publication of these images.
References
- Ilczyszyn A, Ridha H, Durrani AJ. Management of chyle leak post neck dissection: a case report and literature review. J Plast Reconstr Aesthet Surg 2011;64:e223-30. [Crossref] [PubMed]
- Nussenbaum B, Liu JH, Sinard RJ. Systematic management of chyle fistula: the Southwestern experience and review of the literature. Otolaryngol Head Neck Surg 2000;122:31-8. [Crossref] [PubMed]
- Singh M, Deo SV, Shukla NK, et al. Chylous fistula after axillary lymph node dissection: incidence, management, and possible cause. Clin Breast Cancer 2011;11:320-4. [Crossref] [PubMed]
- Rice DC, Emory RE Jr, McIlrath DC, et al. Chylous fistula: an unusual occurrence after mastectomy with immediate breast reconstruction. Plast Reconstr Surg 1994;93:399-401. [Crossref] [PubMed]
- Daggett JD, Watt AW, Smith PD. Chyle leak following right axillary lymph node dissection: A case report and review of current literature. Int J Surg Case Rep 2016;20:68-73. [Crossref] [PubMed]
- Sakman G, Parsak CK, Demircan O. A rare complication in breast cancer surgery: chylous fistula and its treatment. Acta Chir Belg 2007;107:317-9. [Crossref] [PubMed]
- Langford RJ, Daudia AT, Malins TJ. A morphological study of the thoracic duct at the jugulo-subclavian junction. J Craniomaxillofac Surg 1999;27:100-4. [Crossref] [PubMed]
- Delaney SW, Shi H, Shokrani A, et al. Management of Chyle Leak after Head and Neck Surgery: Review of Current Treatment Strategies. Int J Otolaryngol 2017;2017. [Crossref] [PubMed]
- González-Sánchez-Migallón E, Aguilar-Jiménez J, García-Marín JA, et al. Chylous Fistula following Axillary Lymphadenectomy: Benefit of Octreotide Treatment. Case Rep Surg 2016;2016. [Crossref] [PubMed]
- Jain A, Singh SN, Singhal P, et al. A prospective study on the role of octreotide in management of chyle fistula neck. Laryngoscope 2015;125:1624-7. [Crossref] [PubMed]


